Gardasil vaccination. Vaccine "Gardasil": description of the instructions, side effects, complications.
GARDASIL is a vaccine developed by American scientists and aimed at combating human papillomavirus (HPV) types 6, 11, 16 and 18 (HPV types 6 and 11 cause the development of genital warts, and types 16 and 18 lead to the incidence of uterine cancer in women , and men to cancer of the penis and anal area). This is an experimental vaccine, the effectiveness of which has not been proven over many years of use. Information about it is accompanied by a negative stream of comments and negative reviews.
The most common negative information - the opinion that vaccination with Gardasil can lead to infertility - is most likely a rumor.
First, no research results have been published on this subject. And, secondly, if the vaccine only affects the HPV virus, it does not suppress ovulation, cannot affect the functioning of the ovaries, does not affect the endometrium or the reproductive system of a woman as a whole. The vaccine is not a hormonal drug and is not capable of introducing hormonal failure into work female body. If, nevertheless, harm from the use of the vaccine exists, then all organs in the body, and not exclusively reproductive ones, have a risk of suffering.
On the other hand, it is difficult to say whether it makes much sense to vaccinate girls at an early age (9-10 years). First, there is no evidence of studies confirming the safety and effectiveness of vaccination at this age. Secondly, there is reliable information that the work of the vaccine does not exceed 4 years, and then the degree of protection is greatly reduced. A logical question arises about the advisability of vaccinating nine- and ten-year-old children. Bearing in mind, however, that sexual activity in modern teenagers begins after 15 years, exactly when the effect of the vaccine has ceased.
At the same time, advertising for the Gardasil vaccine is a classic disease trade: instilling fear of the disease as a way to push the client to right decision problem, which is quite accessible and relatively quickly and easily implemented.
In our opinion, the attitude towards vaccines, the effect of which has not been studied for 15-20 years and for which there is no reliable information on the ratio of benefits and risks of their use, should be cautious and prudent.
How does Gardasil help in the fight against papillomavirus? How safe is this drug? You can answer these and other questions by reading this article.
A little about the human papillomavirus itself
papillomas on the human bodyThe human papillomavirus is very diverse, as it has about 100 varieties. Some of them are safe for humans, do not cause any serious illnesses, but only cosmetic defects skin in the form of warts; others can provoke the development of deadly ailments, in the form of cancer of the cervix, penis, and anal area. Papillomaviruses are transmitted in the following ways:
- natural sexual contact, as well as through oral and anal contact;
- household, when the virus can enter the human body through damaged areas of the skin;
- from mother to child, during childbirth;
- self-infection, the virus is able to spread from one area of the skin to another.
Due to the multiplicity possible ways transmission of the virus, almost everyone is a carrier of one or another type of HPV. However, the development of viral particles does not always occur, since the immune system suppresses their effect on the body. But if certain malfunctions occur in the immune system, then papillomaviruses immediately begin to multiply, affecting the cells of the skin of the body or genital organs. The latter, in turn, leads to the formation of extremely unpleasant warts, genital warts on the skin of the genitals, dysplasia of the tissues of the cervix and, as a result, to malignant formations on the mucous membrane of the cervix.
The international scientific community of that time searched for a remedy that would help prevent the development of such diseases by destroying the papillomavirus in the early stages. And now the international research organization Merck and Co. has developed the Gardasil vaccine, which blocks the development of the 4 most dangerous papillomaviruses that lead to the development of cancer in women and men. Clinical trials of the vaccine showed a high positive result.
The composition of the vaccine, instructions for use
 GARDASIL vaccine
GARDASIL vaccine The Gardasil vaccine contains in its composition substances that, when entering the human body, contribute to the mobilization of immune forces, thereby enhancing the effectiveness of the drug. The composition of the Gardasil vaccine is as follows:
- proteins that make up the envelope of papillomavirus strain 6,11,16,18. These protein substances, entering the human body, activate the activity of humoral immunity, which produces antibodies against these types of HPV;
- adjuvant - complex connection aluminum hydroxyphosphate sulfate, which enhances the response immune system when viruses enter the body;
- yeast protein;
- alpha-amino acid histidine;
- polysorbate-80;
- medium salt sodium borate.
The composition of this drug does not include mercury-containing substances, strains of live or dead viruses, but only viral protein compounds that, when they enter the human body, do not have the ability to multiply, but only stimulate the immune system to produce antibodies.
Gardasil vaccine can be given to girls and women aged 9 to 26, and boys and boys aged 9 to 17. This vaccine is only a means of prevention, it is powerless for existing diseases caused by papillomavirus 6, 11,16,18. It is best to vaccinate before the onset of sexual activity. In this regard, such an event is held for girls aged 12 and 13 years.
On the this moment nine-valent Gardasil is used, which helps to additionally develop immunity against 5 other types of papillomavirus. These are types such as 31,33,45,52,58. Since 2013, this drug has been approved and registered by 125 countries, in some of which vaccination with this vaccine is included in the national vaccination schedule. 
Vaccination includes three vaccinations, which are given in the deltoid muscle of the shoulder ( intravenous administration prohibited). 2 months after the first vaccination, a second is given, and then 4 months later, a third. A single dose of the drug is 0.5 ml. You can speed up the process of administering the vaccine. After the first injection of the drug, the procedure is repeated after a month and then after three months. Before use, the vial containing the vaccine is shaken and then drawn into a syringe. After shaking, the suspension should be cloudy in color, but if some unknown particles are found in the contents of the ampoule, or the liquid has changed color, then it cannot be used.
Contraindications to the use of the drug
Basically, this drug has no special contraindications, but the introduction of a vaccine is not recommended for the following categories of people:
- whose organisms have already been attacked by strains of papillomaviruses 6,11,16,18. In this case, the vaccine will not work;
- leading an active sex life. The risk of infection with these types of papillomavirus particles increases many times over. It is best to administer the vaccine before the onset of sexual activity;
- if there is an allergy to any of the substances that make up the drug;
- children under 9 years of age;
- if there are malfunctions in the immune system, in this case it is not a fact that it will be able to respond correctly to the introduced vaccine and develop antibodies against papillomavirus;
- problems with the blood clotting process due to a genetic disease - hemophilia,
- pathological conditions caused by thrombocytopenia - a decrease in the number of platelets that are directly involved in blood clotting.
Side effects after drug administration
 In most cases, people normally tolerate the introduction of the vaccine, and there are no serious reactions from the body. However, as with other vaccine administrations, there may be some side effects after using the drug:
In most cases, people normally tolerate the introduction of the vaccine, and there are no serious reactions from the body. However, as with other vaccine administrations, there may be some side effects after using the drug:
- the occurrence of an allergic reaction in the area of vaccine administration. There may be slight reddening of the skin, urticaria;
- fever, weakness, dizziness after vaccination. In some cases there were fainting spells;
- nausea, vomiting, soreness of muscle tissue, head;
- swelling of the face, neck.
In the presence of most of the above symptoms, it is better not to re-vaccinate, in order to avoid worsening the condition. There have been cases of pulmonary embolism - an acute process of blockage of vital blood vessels, which can lead to death as a result of blocking the blood circulation of organ tissues by a detached embolus (thrombus). Given the latter, some people are wary of getting vaccinated with Gardasil. Although, according to experts, with the introduction of any vaccine there are side effects, which in some cases can lead to death.
Remember: the decision to vaccinate with Gardasil is yours alone!
Safety regulations. Use of the drug during pregnancy
 It is important to know that after the start of vaccination, you should try to avoid pregnancy, since vaccinations are prohibited during this period. If conception nevertheless occurred, during the three vaccinations, then it will be possible to resume it only after childbirth. There are no contraindications to vaccination with the drug during the period breastfeeding.
It is important to know that after the start of vaccination, you should try to avoid pregnancy, since vaccinations are prohibited during this period. If conception nevertheless occurred, during the three vaccinations, then it will be possible to resume it only after childbirth. There are no contraindications to vaccination with the drug during the period breastfeeding.
If you still decide to vaccinate with this drug, then it is important to follow some rules:
- before vaccination, it is important to undergo a comprehensive examination of the body, to identify existing diseases in which it may not be worth vaccinating;
- pass smears for a cytological test, which will show the presence of papillomavirus particles of dangerous strains in the body. In this case, it is not worth vaccinating, but if only one of some types of papillomavirus is detected, then the vaccine will be able to protect against others that have not been identified;
- in order to avoid nausea, fainting, dizziness, after the injection, you need to rest for a while. It must be under close supervision medical staff, which in case of development of an anaphylactic reaction or other manifestations on the part of the body will have everything you need;
- within 24 hours, contact of the vaccination site with water should be avoided.
Interaction with other drugs
 After the start of vaccination, you can take other drugs necessary for diseases: anti-inflammatory, analgesics, hormonal agents, vitamins, antibiotics. They do not affect the effectiveness of the drug Gardasil. There is no data on how Gardasil and immunosuppressants are combined.
After the start of vaccination, you can take other drugs necessary for diseases: anti-inflammatory, analgesics, hormonal agents, vitamins, antibiotics. They do not affect the effectiveness of the drug Gardasil. There is no data on how Gardasil and immunosuppressants are combined.
Gardasil can be administered along with other vaccinations. Just in the case of inoculation on the same day, soreness, swelling of the skin area in the place where the injection was made may increase. Some experts argue that after the introduction of Gardasil, you need to wait a month for vaccination with another drug.
The effectiveness of the drug
 According to expert scientists, the Gardasil vaccine can almost 100% protect a person from pathological changes in the tissues of the cervix, which can lead to cancer. The remedy also helps the male population from the appearance of genital warts, cancer of the anal area and other precancerous conditions.
According to expert scientists, the Gardasil vaccine can almost 100% protect a person from pathological changes in the tissues of the cervix, which can lead to cancer. The remedy also helps the male population from the appearance of genital warts, cancer of the anal area and other precancerous conditions.
Before vaccination, it would be good to get reliable information from a specialist. However, even after vaccination of the female part of the population, it is necessary to regularly visit a gynecologist, since many other factors, in addition to papillomaviruses, can provoke the development of female diseases.
Remember that the most important thing for maintaining health is not vaccination, but maintaining healthy lifestyle life, in particular:
- proper nutrition, which includes the use of fortified fruits, vegetables, cereals;
- healthy sleep, positive emotions;
- sex life, must be fully controlled, protected by contraceptives. It is important to avoid frequent change of sexual partners;
- regular medical examination by a gynecologist, urologist, proctologist.
Adhering to such simple rules, you can protect yourself as much as possible from all kinds of diseases, including cancer, and if any disease is detected, it is necessary to start treatment on time, which will increase the chances of being cured even from serious diseases.
Content
One of the fastest growing infections with many strains is human papillomavirus infection. Some of its types can cause skin warts. Others help the emergence and development of genital warts on the mucous tissues of the body. Some forms of this virus, designated by special numbers (these are numbers 6 and 11, 16 and 18), can lead to cancer of the genital organs. Modern medicine has not yet found a way or medicine (vaccination) that completely eliminates papillomavirus. The only option that is now universally available is vaccination.
For vaccination, there are two drugs - Gardasil and Cervarix. In some states, vaccination with these drugs has become mandatory, but in Russia it still remains voluntary. Many people believe that these vaccines are dangerous and cause many side effects, even death.
Before deciding for yourself whether this is true, you need to consult a doctor, undergo a complete medical examination and examination for the presence of a virus. You should read the reviews of people who have already used the Gardasil vaccine in order to understand what you may encounter and it is worth getting vaccinated.
Cervarix and Gardasil
These two vaccines are drugs that supposedly protect against infection with human papillomavirus infection, more precisely, from its specific forms - Cervarix protects against forms 16 and 18. And the Gardasil vaccine, in addition to the first two, also fights against strains 6 and 11. The experiments of medical institutes indicate that these strains lead, under certain conditions, to the development of cancer - 16 and 18 can lead to cervical cancer, 6 and 11 can form a cancerous disease in men. 6 and 11 are less conducive cancerous tumors, but have a stronger effect on the development of genital warts.
Vaccine Gardasil is not a cure for cancer.
This is just a vaccination against several forms of papillomavirus infection (6 and 11, 16 and 18), which can become provocateurs of cervical cancer under certain conditions. Gardasil also fights against warts on the skin near the genitals, which also does not in any way relate to cancer. The Gardasil vaccine does not provide protection if the papillomavirus is already in the human body.
Papillomavirus infection has at least a dozen more strains, and even if vaccination provides protection against these four forms of the virus, the remaining forms will remain dangerous.

Composition of Gardasil
Cervarix and Gardasil are divalent drugs and contain modified synthetic proteins that are similar in structure to the structure of the virus. It should be noted that the drugs are not medicinal. They can only protect against the penetration of papillomavirus, but will not be able to fight an already developing virus. When these vaccines are introduced into the body, the human immune system is activated, which leads to the reproduction of antibodies that protect the body from the development of the virus when it enters the body. They also include aluminum hydroxide, which helps the immune system, sodium chloride, polysorbate and water for injection.
Official statements say that the components of the drug Gardasil are completely safe and harmless. They are stored in sterile vials or syringes in a cool place. One package usually consists of three ampoules - vaccination takes place in three stages, a certain period of time must pass between each capsule.
The cost of one package of Gardasil, consisting of three ampoules, starts from hundreds of dollars. When buying, it is better to immediately pay attention to the storage conditions of the vaccine, since improper storage can not only reduce the strength of the positive impact, but also reverse them, imposing negative symptoms. You can buy the vaccine yourself, you should read the Gardasil instructions before use, but they still need to be vaccinated in sterile medical conditions and only after the full examination.
Application conditions
Since the Gardasil or Cervarix vaccine cannot protect against a virus already present and developing in the body, it is necessary to undergo an examination before using it, to be tested for its presence. It can only be effective for certain groups.
- People whose examination did not reveal the presence of human papillomavirus infection in the body. But even if one of the four possible ones is found, the vaccine can protect against the occurrence of the remaining ones.
- Girls who have not had sexual intercourse - the forms of the virus, under the four numbers indicated above, are often transmitted precisely during sexual intercourse, and during pregnancy it can pass to the fetus through infected organs.

The Gardasil vaccination best case must be completed before the age of 26. The creators of the vaccine are advised to vaccinate children in the time interval from 10 to 12 years. This is due to the fact that at this age children still do not have sexual intercourse, and their immune system works almost perfectly, which helps the drug to act on the body as efficiently as possible.
There are two options vaccination with Gardasil. This standard scheme conducting and accelerated.
Standard scheme carried out in three approaches - three times the injection of the vaccine in half a milliliter. The time interval between the first and second vaccination is two months. Between the second and third - six months.
accelerated scheme differs only in time - the second vaccination is given after a month, the third - two months after the second.
Vaccination with Cervarix is possible only with the standard scheme.
The vaccine is administered intramuscularly. Other options - intravenous, subcutaneous injection - are absolutely not permissible. Usually the vaccine is placed in the upper third of the shoulder. When injected into the muscles, the effect is provided in which the contents of the vaccine are delivered into the blood gradually, in small portions, which helps the gradual production of the necessary antibodies. When, for example, an injection in the buttock is not good option due to the subcutaneous fat layer in that part of the body.
Before giving the first injection, it is better to think about purchasing all three injections at once - the price of the drug is very high, and when deciding to administer the vaccine, it is better to have everything at once than to interrupt the course. An incomplete course does not provide guaranteed virus blocking.
After vaccination you need to stay for half an hour under the supervision of specialists. And during the whole subsequent day, the place where the injection was placed must not be wetted.
The papilloma virus is transmitted not only through sexual intercourse. It can also happen due to non-sterile medical instruments. A child can get an infection while passing through the birth canal if his mother was infected before conception. The disease may not show up. long time, slowly developing, and show itself only at times when immunity is most weakened - during the course of other diseases. The vaccine will not be of any benefit unless a complete examination is made before the injection. Because, for example, if a person already has the papilloma virus in his body, then the vaccine and the money spent on it will be wasted - since it is not a cure for the virus, but only protection against its penetration.
One of the disadvantages of this vaccine is the fact that no long-term study of its effects has been conducted. And also there is no publicly available information base containing data on the impact, possible consequences and complications on the body of the vaccine from the people in whom it was used. For a long time there is no data that speaks about the quality of vaccination in the future, which raises the question of whether it is worth putting such a vaccine and whether it is worth making it mandatory throughout the country.
Contraindications and consequences
Contraindications for Gardasil vaccination are:
- you can not vaccinate if you are allergic to any of the components of the drug;
- during pregnancy;
- in acute inflammatory processes or infections - first you need to be completely cured;
- Cervarix should also not be given to mothers who are breastfeeding;
- in cases where the infection is already in the body;
- children until they reach the age of nine;
- women and men over 45;
- with defects in the blood coagulation system.
It is believed, according to official sources, that the Gardasil vaccine is the most effective in the fight against papillomavirus and slightly less effective in the fight against genital warts. The remedy is advertised as completely safe, but there are many stories about the harm caused by this vaccine. Some believe that this vaccine has a very serious consequences such as infertility.
Complications of vaccination recorded in the system of registration of consequences when using the vaccine are as follows:
- fainting and lupus
- thrombosis,
- cardiac arrest and stroke
- vasculitis,
- ends the list with death.
Comparing Gardasil with other vaccines, we can say that it gives a much more negative effect and causes Negative consequences. When it is used, body temperature rises, muscle pain, headaches, general malaise and dizziness are characteristic. And each next injection is perceived many times worse than the previous one. It can also be said that there were more cases of serious complications, since according to statistics, only a small part of cases are recorded in the system for registering the effects of vaccination.
The official website of the manufacturer does not provide statistics and information about previously vaccinated people. But there is information that the serious consequences of Gardasil appeared precisely after the vaccination of girls in adolescence. From the use of this vaccine, several deaths and more than two thousand cases of severe complications have been registered. In a vaccinated person, health deteriorates, seizures and fainting begin. A deterioration in appetite is fixed, the effectiveness of the immune system is reduced. Because of the drug, hair falls out, and even there is an effect on the mental state - a person can become more aggressive, poorly control his emotional state.
The developers of the drug do not link this data to their vaccine. But on medical records people who have registered complications, it was clear that nothing like this had ever been observed before.
But at the same time, even with the effectiveness of the drug, this does not mean that it is worth canceling the mandatory tests for the presence of human papillomavirus infection. It was only through the fact that this analysis became mandatory in a number of countries that the number of cases of infection was reduced.
It makes even less sense to use the drug for young boys and girls, because the papillomavirus itself is a virus that a healthy children's body has been successfully fighting on its own for several years.
No clinical trials have been conducted on Gardasil, there is no evidence that it is effective and that its protection against the virus will last for many years. Manufacturers say that protection can last four years. Participants in the manufacturer's trials to prove the effectiveness of Gardasil had to introduce the fourth ampoule to the subjects, to demonstrate the reproduction of antibodies that fight the papillomavirus in the body. Information about the vaccine warns that the drug does not guarantee complete protection.
When sending any medicinal preparations to the market, the main factor in testing should be the safety of the product.
Gardasil should have been tested more thoroughly before marketing authorization was given.
Before making a decision whether to vaccinate Gardasil or Cervarix, you need to consult with specialists, oncologists.
You also need to find out about the patient's genetic predisposition to cancer. You should undergo a complete examination and check for the presence in the body of certain strains of human papillomavirus infection.
Reviews of medicines, description, medicines, rating of medicines, instructions for use, user reviews, special instructions, side effects, overdose, use, indications
reviews 1
Gardasil- vaccine against human papillomavirus quadrivalent recombinant (types 6,11,16,18).The quadrivalent human papillomavirus (HPV) vaccine is a mixture of highly purified virus-like particles (VLPs) of recombinant major capsid protein (L1) of HPV types 6,11,16 and 18. L1 proteins are produced by separate fermentation in recombinant Saccharomyces cerevisiae CANADA 3C-5 ( Strain 1895) and form VHF by self-assembly. HPS for each type are purified and adsorbed on an aluminum-containing adjuvant (amorphous aluminum hydroxyphosphate sulfate).
Conducting a full course of vaccination leads to the formation of specific antibodies to four types of HPV - 6,11,16 and 18 - in a protective titer in more than 99% of those vaccinated. Protection against genital cancers, precancerous dysplasia and genital warts induced by specific types of HPV was maintained for at least 54 months after the completed vaccination course.
Based on studies conducted in women aged 16 to 45 years, a high profile of the efficacy, safety and immunogenicity of the Gardasil vaccine has been confirmed. In girls and boys from 9 to 15 years of age, clinical studies were conducted to study the safety and immunogenicity, and based on immune bridging, the effectiveness of the vaccine was shown.
Indications for use:
Vaccine Gardasil is indicated for use in children and adolescents aged 9 to 15 and women aged 16 to 45 to prevent:
- precancerous dysplastic conditions (cervix, vulva and vagina) and cervical cancer caused by oncogenic types of human papillomavirus (HPV),
- genital warts of the external genital organs (condiloma acuminate) etiologically associated with specific types of HPV.
Vaccine Gardasil should be used in accordance with the doctor's prescription for the prevention of diseases caused by human papillomavirus types 6,11, 16, 18, and to a lesser extent, diseases caused by other types of HPV.
Mode of application:
Vaccine Gardasil injected intramuscularly into the deltoid muscle or the upper outer surface of the middle third of the thigh.
Do not administer intravenously.
A single dose for all age groups is 0.5 ml.
The recommended vaccination course consists of 3 doses and is carried out according to the scheme (0-2-6 months):
The first dose - on the appointed day.
The second dose is 2 months after the first.
The third dose is 6 months after the first.
A vaccination schedule is allowed, in which the second dose is administered 1 month after the first vaccination, and the third 3 months after the second vaccination. If the interval between vaccinations is violated, the vaccination course is considered completed if three vaccinations are carried out within 1 year.
The need for revaccination has not been established.
If the first dose of Gardasil vaccine was used for vaccination, then the full course of vaccination should be carried out using the Gardasil vaccine.
Before use, the vial / syringe with the vaccine is shaken until a homogeneous cloudy suspension is obtained. Loss of homogeneity, the presence of inclusions and foreign particles, a change in the color of the suspension indicate the unsuitability of the vaccine.
The vaccine syringe is for single use only and in one person only. You should enter the entire recommended dose - 0.5 ml.
The opening of the vials and the vaccination procedure is carried out with strict observance of the rules of asepsis and antisepsis. The injection site before and after the injection is treated with 70% alcohol.
Using single dose vials of vaccine
Withdraw 0.5 ml of suspension from the single dose vial of the vaccine with a sterile needle into disposable syringe. Enter the entire dose. Throw away the vaccine vial.
Use of syringes with a single dose of vaccine
Inject the entire contents of the syringe completely.
Use an inserted needle to administer the vaccine. If you wish to use a different one, make sure that the needle is securely connected to the syringe and that it is no longer than 2.5 cm, which is necessary condition for correct operation protective device.
Remove the cap from the syringe. While pressing both anti-rotation tabs, secure the syringe, and attach the Luer lock by turning it clockwise. Remove the protective cap from the needle.
When administering the injection as above, depress the plunger while holding the syringe firmly under your fingertips and inject the entire dose. The safety device will not work unless the ENTIRE dose is delivered. Remove the needle. Lower the plunger and let the syringe move up until the needle is completely closed. Discard the syringe in a sharps container.
To document vaccinations, separate the removable labels by pulling them slowly.
Side effects:
The following vaccine-related adverse reactions have occurred in individuals receiving Gardasil, in 1% of cases and more often than in persons who were given placebo.
Musculoskeletal disorders and connective tissue damage.
Often: pain in the limbs.
General reactions and reactions at the injection site.
Often: pyrexia.
The following local reactions occurred in the Gardasil group compared to any formulation containing aluminum hydroxyphosphate sulfate amorphous adjuvant or compared to the placebo solution group.
Very common: redness, pain and swelling.
Often: itching, hematoma.
Most local reactions were of mild severity.
In addition, bronchospasms, as serious side effects, were very rare.
There have been several spontaneous reports of adverse reactions during post-marketing use of the Gardasil vaccine. Since these reactions have been reported on own will population, it is not possible to reliably estimate their frequency or establish a causal relationship with the use of the vaccine.
Blood and lymphatic system disorders: lymphadenopathy, idiopathic purpura.
Violations nervous system: dizziness, acute primary idiopathic polyradiculoneuritis, headache, Guillain-Barré syndrome, acute disseminated encephalomyelitis, syncope, sometimes accompanied by tonic-clonic convulsions.
Gastrointestinal disorders: nausea, vomiting.
Musculoskeletal injuries: arthralgia, myalgia.
General reactions: asthenia, fatigue, chills, discomfort.
Immune System Disorders: Hypersensitivity reactions including anaphylactic/anaphylactoid reactions, bronchospasm and urticaria.
Contraindications:
Hypersensitivity to the active components and excipients of the vaccine. If symptoms of hypersensitivity occur after administration of the vaccine Gardasil the introduction of a subsequent dose of the vaccine is contraindicated.
Bleeding disorders due to hemophilia, thrombocytopenia or anticoagulants are a relative contraindication to intramuscular administration of the Gardasil vaccine, unless the potential benefits of vaccination greatly outweigh the risks associated with it. If the choice is made in favor of vaccination, measures must be taken to reduce the risk of post-injection hematoma formation.
If the patient has an acute severe febrile illness, then the introduction of the Gardasil vaccine should be postponed. However, the presence of a mild infection or a slight rise in body temperature is not a contraindication to vaccination.
Pregnancy:
There is no evidence that the introduction of the vaccine Gardasil has an undesirable effect on fertility, pregnancy or the fetus, and which would cast doubt on its safety.
There are no specially designed and well-controlled studies in pregnant women. Data on the use of the Gardasil vaccine during pregnancy and on the potential impact of the Gardasil vaccine on female reproductive function and on the fetus in pregnant women are insufficient to recommend the use of the vaccine during pregnancy.
Patients should be warned about the need to protect against pregnancy during the course of vaccination, and if pregnancy occurs, vaccination should be postponed until it is completed.
Clinical trials testing the efficacy, immunogenicity, and safety of Gardasil vaccine in breastfeeding mothers and infants have shown that Gardasil vaccine can be administered to breastfeeding women.
Interactions with other drugs:
vaccine Gardasil can be administered simultaneously (in a different site) with a recombinant hepatitis B vaccine, a meningococcal vaccine conjugated with diphtheria toxoid and an inactivated vaccine against diphtheria, tetanus, whooping cough (acellular component), poliomyelitis.
The use of analgesics, anti-inflammatory drugs, antibiotics and vitamin preparations did not affect the efficacy, immunogenicity and safety of the vaccine.
Application hormonal contraceptives did not affect the efficacy, immunogenicity or safety of the Gardasil vaccine.
Inhaled, topical and parenteral steroids did not affect the immunogenicity and safety of the Gardasil vaccine.
Data on the simultaneous use of systemic immunosuppressants and the Gardasil vaccine are not available.
Overdose:
There have been reports of cases of vaccine administration Gardasil in doses higher than those recommended. In general, the nature and severity of adverse events with overdose were comparable to those with the introduction of the recommended single doses of the Gardasil vaccine.
Storage conditions:
vaccine Gardasil should be stored at a temperature of 2 to 8 ° C, protected from light.
Do not freeze.
Keep out of the reach of children.
Gardasil should be administered as soon as possible after removal from the refrigerator. Gardasil may be kept out of the refrigerator (at 25°C or below) for up to 72 hours.
Release form:
Vial Primary packaging: 1 dose (0.5 ml) is placed in a sterile vial (capacity 3 ml) and type 1 tubular glass. The bottle is sealed with a Teflon-coated chlorobutyl stopper, under an aluminum rim and closed with a snap-on plastic cap.
Secondary packaging: 1 or 10 bottles in a carton box along with instructions for use.
Disposable syringe Primary packaging: 1 dose (0.5 ml) in a disposable sterile syringe (1.5 ml capacity) made of borosilicate glass. Syringe with or without safety device, equipped with a polycarbonate adapter, a protective bromobutyl cap and a piston closed with a butyl rubber stopper, coated with silicone.
1 disposable sterile pre-filled syringe complete with 1 or 2 sterile needles (or without needles) is placed in a blister pack with a cap.
Secondary packaging: 1 or 10 disposable sterile syringes, sealed in a blister pack with a lid, in a carton box along with instructions for use.
Composition:
One dose (0.5 ml) Gardasil contains:
Active substances: recombinant antigens: human papillomavirus L1 protein in the following amounts: type 6 (20 µg), type 11 (40 µg), type 16 (40 µg), type 18 (20 µg).
Excipients: amorphous aluminum hydroxyphosphate sulfate - 225 mcg, sodium chloride - 9.56 mg, L-histidine - 0.78 mg, polysorbate-80 - 50 mcg, sodium borate - 35 mcg, water for injection.
Does not contain preservatives or antibiotics.
Additionally:
When deciding on vaccination, it is necessary to compare possible risk from prior HPV infection and the potential benefit of vaccination.
Vaccine Gardasil is not intended for the treatment of cervical, vulvar or vaginal cancer, CIN, VIN, or VaIN, or active condylomatosis and is only for prophylactic use. The vaccine is preventive and is designed to prevent infection with types of HPV that the patient does not have. The vaccine does not affect the course of active infections caused by HPV. As with the introduction of any other vaccine, when using Gardasil, not all vaccinated people manage to get a protective immune response. The drug does not protect against sexually transmitted diseases of another etiology.
Therefore, vaccinated patients should be advised to continue using other preventive protective measures.
Subcutaneous and intradermal administration of the vaccine has not been studied and is therefore not recommended.
As with any injectable vaccine, you should always have the appropriate medicines in case of development of a rare anaphylactic reaction to the introduction of a vaccine and emergency and anti-shock therapy. Immediately after the introduction of the vaccine for 30 minutes, the patient is under medical observation in order to timely detect post-vaccination reactions and complications and provide emergency assistance. Syncope can occur with any vaccination, especially in adolescents and young women.
The decision to administer the drug or delay vaccination due to a current or recent illness accompanied by fever, to a greater extent depends on the etiology of the disease and severity.
In individuals with impaired immune system reactivity due to the use of immunosuppressive therapy (systemic corticosteroids, cytotoxic antimetabolites, alkylating agents), a genetic defect, human immunodeficiency virus (HIV) infection, and other causes, the protective effect may be reduced.
Gardasil vaccine should be administered with caution to persons with thrombocytopenia and any bleeding disorders, because after intramuscular injection these individuals may bleed.
Health care staff are required to provide all relevant vaccination and vaccine information to patients, parents and caregivers, including information on benefits and associated risks.
Those who are vaccinated should be advised to report any adverse reactions to their doctor or nurse and that vaccination does not replace or replace routine screening exams. To achieve effective results, the course of vaccination must be completed completely, if there are no contraindications for this.
Gardasil (Gardasil) - the world's first vaccine that can prevent cancer and cervical dysplasia, the occurrence of genital warts and other pathologies caused by the papillomavirus. The human papillomavirus (HPV is often used) is one of the most common sexually transmitted infections, especially insidious and dangerous because it often long time is asymptomatic.
There is evidence that up to half of humanity is infected with this virus during a lifetime. More than 100 strains of the virus are known to science. different influence on the human body, the most dangerous of which in terms of oncogenicity are types 16 and 18.
The main let HPV infection is through sexual contact, but there is a possibility of infection in the household way. Caused by different strains of the virus, cervical cancer in terms of frequency of occurrence is in the "honorable" second place among malignant oncological pathologies in women, and mortality from this type of cancer is second only to breast cancer. Due to the prevalence of the problem, the new vaccine has become a real sensation in the global pharmaceutical market.
The recombinant (“killed”, does not contain live viruses) 4-valent vaccine Gardasil, produced by the German company Merck & Co, contains highly purified virus-like particles of the main protein of 6, 11, 16 and 18 strains of the papillomavirus. The first two types of HPV cause up to 90% of genital warts in men and women, the last two - up to 70% of reported cases of malignant tumors of the cervix. HPV can also provoke cancers of the vulva in both men and women, significantly increases the risk of oncological diseases of the larynx, is the cause of various precancerous conditions, and also causes such a pathology that is difficult to treat, such as recurrent papillomatosis in children and adults.
Immunization schedule with Gardasil vaccine
Gardasil is recommended for vaccination of children from 9 years of age and boys and girls up to 17 years of age. After the age of 18, young women are subject to vaccination. The vaccine is most effective before the onset of sexual activity and possible HPV infection, and therefore the manufacturer's recommended age for vaccination is 12-13 years.
Teenagers are an important group for HPV immunization, as age group Every fourth person has been infected with the virus for 15-25 years.
Gardasil vaccination is done three times within six months (scheme 0 - 2 - 6), i.e. the first dose - on the prescribed day, 2 months. after the second vaccination, and the final dose after 6 months. from the first injection. The vaccine can be used for emergency immunization according to the scheme 0 - 1 - 4. In case of violation of the established vaccination schedule, the Gardasil vaccination course is considered completed if all three doses of the vaccine have been received within 1 year. Revaccination Gardasil does not require. After completion of the vaccination course, the production of specific antibodies to the listed HPV strains is recorded in more than 98% of those vaccinated, thus Gardasil vaccination provides clinically effective protection and causes the development of a long-term significant protective within 8 years.
 The vaccine may cause side effects of comparable frequency and similar symptoms to all other vaccines. Usually this is weakness, induration and swelling in the place where the vaccine was injected, headache, nausea, a slight rise in temperature are possible. These symptoms do not need treatment and disappear without a trace after a few days. There are reviews of an acute allergic reaction to the components of the drug, in which case the vaccine should no longer be used. A conditional contraindication for the use of the Gardasil vaccine is thrombocytopenia (a decrease in the number of platelets) and any other blood clotting disorders, due to the risk internal bleeding or thrombosis after injection.
The vaccine may cause side effects of comparable frequency and similar symptoms to all other vaccines. Usually this is weakness, induration and swelling in the place where the vaccine was injected, headache, nausea, a slight rise in temperature are possible. These symptoms do not need treatment and disappear without a trace after a few days. There are reviews of an acute allergic reaction to the components of the drug, in which case the vaccine should no longer be used. A conditional contraindication for the use of the Gardasil vaccine is thrombocytopenia (a decrease in the number of platelets) and any other blood clotting disorders, due to the risk internal bleeding or thrombosis after injection.
The vaccine is incompatible with alcohol, which should be avoided a few days before and after vaccination.
Does not interfere with pregnancy, and theoretically can be used during this period. However, due to the lack experimental studies about the possible negative impact on the development of the fetus, it is better to postpone the Gardasil vaccination and do it after childbirth. The vaccine can be used during breastfeeding.
Efficiency, reviews, conclusions
Gardasil has been registered and approved for use in Russia since 2006, and from the very moment when the first vaccination was made, it caused numerous reviews. A few years ago, this vaccine was perhaps the most discussed both on specialized medical resources and on women's forums. Since the opinions of the participants in discussions about the vaccine sometimes differ polarly, in the flow of conflicting information it is often very difficult to make a choice.
Reviews of doctors about the Gardasil vaccine are also of a different nature, some are strong supporters of vaccination, others comment on the mandatory immunization much more cautiously. On the one hand, there are more than convincing results of numerous clinical studies confirming the high reliability and effectiveness of vaccination. On the other hand, there are numerous reviews that the vaccine causes infertility and the development of other pathologies of the reproductive system, that Gardasil has not been sufficiently studied or studied inappropriately (in particular, a real placebo was not used in clinical experiments).
Such radically opposite reviews are not uncommon when discussing other vaccines. It is important to understand that Gardasil is not a cancer vaccine, much less a panacea for it. This vaccine is designed to protect mainly against the two most dangerous strains of the virus that are most likely to provoke cervical cancer. There are other strains of papillomavirus that can provoke oncological pathologies that Gardasil does not cover in any way, so regular PAP smear (a cytological smear to determine precancerous or cancer cells) remains fundamentally important event in antenatal clinics, which allows diagnosing precancerous changes at an early stage.
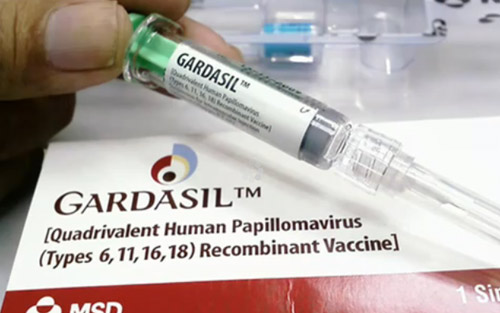 In any case, in search of information in Internet sources, it is preferable to choose reviews of reputable doctors. It should always be remembered that despite advertising and positioning by the manufacturer in the media, any vaccine, and Gardasil is no exception - not a wonderful harmless essence, but a serious drug, the use of which carries quite certain risks. Before deciding whether to vaccinate with Gardasil, it is useful to weigh the possible risks of prior HPV infection against the potential benefits.
In any case, in search of information in Internet sources, it is preferable to choose reviews of reputable doctors. It should always be remembered that despite advertising and positioning by the manufacturer in the media, any vaccine, and Gardasil is no exception - not a wonderful harmless essence, but a serious drug, the use of which carries quite certain risks. Before deciding whether to vaccinate with Gardasil, it is useful to weigh the possible risks of prior HPV infection against the potential benefits.
Where to buy and how much?
The Gardasil vaccine is available in three forms- single dose vial, disposable syringe, disposable syringe with protective device, and is an opaque suspension white color. All release forms are interchangeable. The vaccine is administered in the same dosage regardless of the gender and age of the patient.
Gardasil is given intramuscularly into the deltoid muscle of the shoulder or the anterolateral part of the thigh. The vaccine can be combined with a hepatitis B vaccine (in this case, the vaccine must be administered in different parts of the body).
In our country, the Gardasil vaccination is not included in the National Calendar and is not mandatory, so if you want to get vaccinated against HPV, you will have to purchase the drug at your own expense. The vaccine is relatively expensive - the price of Gardasil, depending on the form of release, starts at several thousand rubles.
The vaccine is distributed through the pharmacy network and dispensed by prescription. The price of Gardasil varies significantly depending on the region of residence. The cost of the vaccine is slightly lower when buying in bulk or through online pharmacies. The only analogue of the Gardasil vaccine is the drug Cervarix, which was registered in 2008, the price of which is slightly lower (protects only against 16 and 18 types of HPV).