Functional regions of the tRNA. Structure and functions of tRNA, features of amino acid activation
70-90N | secondary page - cloverleaf | CCA 3" const for all tRNA |
the presence of thymine, pseudouridine-psi, digirouridine DGU in the D-loop - protection against ribonucleases? long-lived | A variety of primary structures of tRNA - 61 + 1 - by the number of codons + formylmethionine tRNA, the cat's anticodon is the same as that of methionine tRNA. Variety of tertiary structures - 20 (according to the number of amino acids) | recognition - the formation of a covalent bond m-y tRNA and act | aminoacyl-tRNA synthetases attach acts to tRNA
The function of tRNA is to transfer amino acids from the cytoplasm to the ribosomes, in which protein synthesis occurs.
tRNAs that bind one amino acid are called isoacceptor.
In total, 64 different tRNAs simultaneously exist in a cell.
Each tRNA pairs only with its own codon.
Each tRNA recognizes its own codon without the involvement of an amino acid. The amino acids bound to the tRNA were chemically modified, after which the resulting polypeptide, which contained the modified amino acid, was analyzed. Cysteinyl-tRNACys (R=CH2-SH) was reduced to alanyl-tRNACys (R=CH3).
Most tRNAs, regardless of their nucleotide sequence, have a cloverleaf-shaped secondary structure due to the presence of three hairpins in it.
Structural features of tRNA
There are always four unpaired nucleotides at the 3 "end of the molecule, and three of them are necessarily CCAs. The 5" and 3" ends of the RNA chain form an acceptor stem. The chains are held together due to the complementary pairing of seven nucleotides 5" - end with seven nucleotides located near the 3 "end. 2. All molecules have a T? C hairpin, so designated because it contains two unusual residues: ribothymidine (T) and pseudouridine (? The hairpin consists of a double-stranded stem of five paired bases, including the G-C pair, and a loop of seven nucleotides in length.
at the same point in the loop. 3. In an anticodon hairpin, the stem is always represented by a family of paired
grounds. The triplet complementary to the related codon, the anticodon, is located in the loop.
le, consisting of seven nucleotides. An invariant ura-
cyl and a modified cytosine, and a modified purine adjoins its 3 "end, as a rule
adenine. 4. Another hairpin consists of a stalk three to four pairs of nucleotides long and a variable loop
size, often containing uracil in a reduced form - dihydrouracil (DU). The nucleotide sequences of the stems, the number of nucleotides between the anticodon stem and the T?C stem (variable loop), as well as the size of the loop and the localization of dihydrouracil residues in the DU loop vary most strongly.
[Singer, 1998].
Tertiary structure of tRNA
L-shaped structure.
Attachment of amino acids to tRNA
In order for an amino acid to form a polypeptide chain, it must be attached to tRNA by the enzyme aminoacyl-tRNA synthetase. This enzyme forms a covalent bond between the amino acid carboxyl group and the ribose hydroxyl group at the 3' end of tRNA with the participation of ATP. Aminoacyl-tRNA synthetase recognizes a specific codon not because of the presence of an anticodon on the tRNA, but by the presence of a specific recognition site on the tRNA.
In total, there are 21 different aminoacyl-tRNA synthetases in the cell.
Joining takes place in two stages:
1. The carboxyl group of an amino acid is attached to ATP a-phosphate. The resulting unstable aminoacyl adenylate is stabilized by binding to the enzyme.
2. Transfer of the aminoacyl group of aminoacyl adenylate to the 2' or 3'-OH group of the terminal ribose of tRNA
Some aminoacyl-tRNA synthetases consist of a single polypeptide chain, while others consist of two or four identical chains, each with a molecular weight of 35 to 115 kDa. Some dimeric and tetrameric enzymes are composed of two types of subunits. There is no clear correlation between the size of the enzyme molecule or the nature of its subunit structure and specificity.
The specificity of an enzyme is determined by its strong binding to the acceptor end of tRNA, the DU region, and the variable loop. Some enzymes do not seem to recognize the anticodon triplet and catalyze the aminoacetylation reaction even when the anticodon is altered. However, some enzymes show reduced activity in relation to such modified tRNAs and add the wrong amino acid when replacing the anticodon.
70-90n | secondary page - cloverleaf | CCA 3" const for all tRNA |
the presence of thymine, pseudouridine-psi, digirouridine DGU in the D-loop - protection against ribonucleases? long-lived | A variety of primary structures of tRNA - 61 + 1 - by the number of codons + formylmethionine tRNA, the cat's anticodon is the same as that of methionine tRNA. Variety of tertiary structures - 20 (according to the number of amino acids)
There are two types of tRNA binding methionine tRNAFMet and tRNAMMet in prokaryotes and tRNAIMet and tRNAMMet in eukaryotes. Methionine is added to each tRNA using the appropriate aminoacyl-tRNA synthesis. methionine attached to tRNAFMet and tRNAIMet is formed by the enzyme methionyl-tRNA-transformylase to Fmet-tRNAFMet. tRNAs loaded with formylmethionine recognize the initiation codon AUG.
Literature:
Unfortunately, there is no bibliography.
Textbook. Despite the fact that tRNA is much smaller, a story about its structure, features, and functioning deserves a separate chapter.
So, tRNA is an “adapter”, which recognizes the three-letter sequence of the genetic code at one end, matching it with the only corresponding amino acid fixed at the other end of the tRNA. At the end of the transfer RNA that touches the messenger RNA, there are 3 nucleotides that form anticodon. Only if the anticodon is complementary to the mRNA region can the transfer RNA join it. But even in this case, tRNA cannot join mRNA on its own; it needs the help of the ribosome, which is the site of their interaction, as well as an active participant in translation. For example, it is the ribosome that creates bonds between amino acids brought by tRNA, forming a protein chain.
The structural features of tRNA are determined by the genetic code, that is, the rules for constructing a protein according to a gene that the transfer RNA reads. This code works in every living creature on Earth: the creation of a virus is written with the same three-letter codons that are used to write the "assembly instructions" of a dolphin. It has been experimentally verified that the genes of one living creature, placed in the cell of another, are perfectly copied and translated into proteins that are indistinguishable from the genes reproducing in the cells of the host. The uniformity of the genetic code is the basis for the production of modified E. coli by colonies of insulin and many other human enzymes that are used as drugs for people whose bodies are not able to produce them, or produce insufficiently. Despite the obvious difference between humans and E. coli, human proteins are easily created from human blueprints using an E. coli copier. Not surprisingly, the transfer RNAs of different creatures differ very little.

Each codon from this list, except for three stop codons, signaling the completion of translation, should be recognized by the transfer RNA. Recognition is carried out by attaching an anticodon to messenger RNA, which can only bind to one codon from the list, so tRNA can recognize only one codon. This means that there are at least 61 types of these molecules in the cell. In fact, there are even more of them, since in some situations for reading messenger RNA it is not enough just to have the right anticodon: other conditions are required, in accordance with which a special, modified tRNA is created.
At first glance, such a variety of tRNAs should significantly complicate the translation process: after all, each of these molecules will check the matrix RNA codon substituted for it by the ribosome for compliance with its anticodon - it would seem that so much pointless mechanical work, so much wasted time and energy. But as a result of evolution, cellular mechanisms have also been formed that prevent this problem. For example, the amount of tRNA of each species in a cell corresponds to how often the amino acid carried by that species is found in the proteins being built. There are amino acids that are rarely used by the cell, and there are those that are often used, and if the number of tRNAs carrying them were the same, this would greatly complicate the assembly of proteins. Therefore, there are few "rare" amino acids and their corresponding tRNAs in the cell, while frequently occurring amino acids are produced in large quantities.

With such a variety of tRNA molecules, they are all very similar, therefore, considering their structure and functions, we will mainly study the features common to all species. When you look at the 3D layout of tRNA, it looks like a dense pile of atoms. It seems incredible that this intricately coiled molecule is the result of the folding of a long chain of nucleotides, but that is how it is formed.
It is possible to trace the stages of its formation, starting from the very first: the compilation of a nucleotide sequence by RNA polymerase in accordance with the gene containing information about this transfer RNA. The order in which these nucleotides follow each other and their number is called primary structure of tRNA. It turns out that it is the primary structure of tRNA that is encoded in the gene read by RNA polymerase. In general, the primary structure is a sequence of relatively simple molecules of the same type, of which a more complex, folded polymer molecule is composed. For example, the primary structure of a protein molecule is the simple sequence of its constituent amino acids.

Any chain of nucleotides cannot be in an unfolded state in a cell, simply stretched out in a line. There are too many positively and negatively charged parts at the edges of the nucleotides, which easily form hydrogen bonds with each other. How the same bonds are formed between the nucleotides of two DNA molecules, connecting them into a double helix, is described in, and you can get into details about hydrogen bonds. Hydrogen bonds are less strong than the bonds between atoms in molecules, but they are enough to twist the tRNA thread intricately and keep it in that position. At first, these bonds are formed only between some nucleotides, folding the tRNA into a clover-leaf shape. The result of this initial folding is called secondary structure tRNA. The diagram on the left shows that only some nucleotides are linked by hydrogen bonds, while others remain unpaired, forming rings and loops. Differences between the secondary structure of different types of tRNA are due to differences in their primary structure. This manifests itself in different lengths of "clover leaves" or "stalk" due to different lengths of the initial chain of nucleotides.
Another difference in the primary structure of different tRNAs is that only in some positions they have the same nucleotides (in the diagram above they are marked with the first letters of their names), while most of the nucleotides in different tRNAs differ from each other. The scheme above is common to all tRNAs, so different nucleotides are marked with numbers.
The main functional parts of tRNA are:
=) anticodon, that is, the nucleotide sequence that is complementary to a single codon of messenger RNA located on anticodon hairpin
=) acceptor end to which an amino acid can be attached. It is located on the opposite side of the anticodon hairpin.
In reality, not a single tRNA looks like it does in the secondary structure diagram, because only some nucleotides joined together to form it, while the rest remained unpaired. Due to the formation of hydrogen bonds between nucleotides from different parts of the clover leaf, it folds further into a much more complex tertiary structure in the shape of an L. You can understand exactly how the different parts of the secondary structure curved to form the tertiary structure by matching the colors in their diagrams below. The anticodon hairpin, marked in blue and gray, remains at the bottom (it is worth remembering that this “bottom” is conditional: it is convenient to depict tRNA in this spatial orientation in protein translation schemes), and the acceptor end (yellow) is bent to the side.

This is what tRNA looks like when it is ready to attach an amino acid. tRNA is not able to combine with the amino acid on its own, this requires the participation of a special enzyme: aminoacyl-tRNA synthetases. The number of synthetase types in a cell coincides with the number of tRNA types.
The uniformity of the shape of all types of tRNA is necessary so that the ribosome can recognize any of them, facilitate their docking with mRNA, and move within itself from one site to another. If different types of tRNA were significantly different from each other, this would make the work of the ribosome extremely difficult, critically reducing the rate of protein synthesis. Natural selection thus aims to make tRNAs similar to each other. But at the same time, there is another factor that requires the existence of noticeable differences between different types of tRNA: after all, it is necessary to recognize each type and attach to it the only corresponding amino acid. Obviously, these differences should be noticeable, but not too significant, so that the work of recognizing tRNA species turns into a jewelry process. And it is precisely this that is carried out by aminoacyl-tRNA synthetases: each of them can bind to only one of the 20 amino acids and attach it precisely to those types of tRNA that correspond to this amino acid. From the table with the genetic code, it can be seen that each amino acid is encoded by several nucleotide sequences, therefore, for example, all four tRNAs with anticodons CGA, CGG, CGU and CGC will be recognized by the same synthetase that attaches alanine to them. Such tRNAs processed by one synthetase are called related.
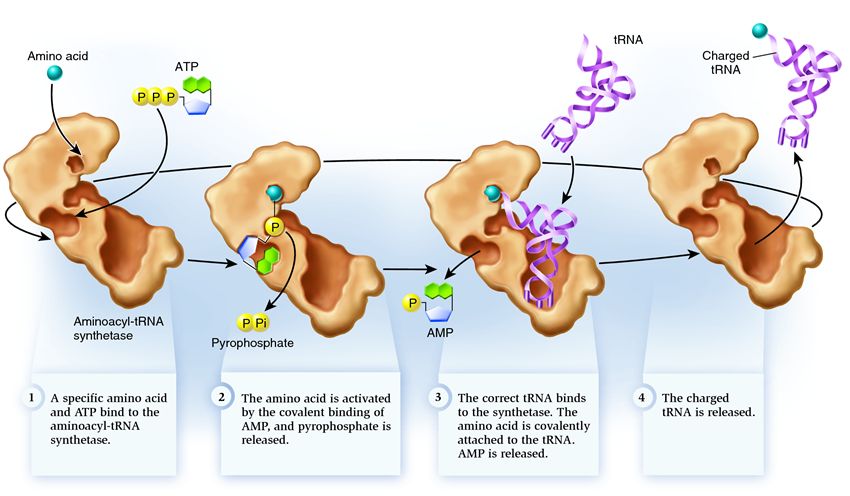
Synthetase belongs to a group of enzymes whose function is to bind to separately existing molecules and combine them into one:
1 . synthetase connects an amino acid and an ATP molecule. Two phosphate groups break away from ATP, releasing the energy needed for the following activities. The adenosine monophosphate (AMP) remaining from the destroyed molecule attaches to the amino acid, preparing it for connection with the acceptor hairpin.
2 . synthetase attaches to itself one of the related tRNAs corresponding to this amino acid.

At this stage, the conformity of the transfer RNA to the synthetase is checked. There are several ways of recognition, and each synthetase has a unique combination of them. At least one anticodon nucleotide is involved in the interaction between synthetase and tRNA. The acceptor hairpin also needs to be checked: the presence of specific nucleotides on it that are common to related tRNAs corresponding to the desired amino acid is determined. Nucleotides from other parts of the tRNA can also participate in matching by binding to certain synthetase sites. The wrong tRNA may match the desired one in some way, but due to incomplete matching, it will join the synthetase slowly and loosely, easily falling off. And the correct tRNA will stick to the synthetase quickly and firmly, as a result of which the structure of the synthetase changes, starting the process aminoacylation , that is, the attachment of an amino acid to tRNA.

3 . aminoacylation consists in replacing the AMP molecule attached to the amino acid with a tRNA molecule. After this replacement, AMP leaves the synthetase and tRNA is held up for one last amino acid check. If the attached amino acid is recognized as incorrect, it will be detached from the tRNA, the amino acid's place in the synthetase will be empty, and another molecule can join there. The new amino acid will go through the stages of connection with ATP and tRNA, and will also be tested. If no mistakes were made, the amino acid-charged tRNA is released: it is ready to play its role in protein translation. And the synthetase is ready to attach new amino acids and tRNAs, and the cycle will begin anew.
A lot depends on the correct operation of the aminoacyl-tRNA synthetase: if a failure occurs at this stage, then the wrong amino acid will be attached to the tRNA. And it will be built into the protein growing on the ribosome, because tRNA and the ribosome do not have the function of checking the correspondence of the codon and amino acid. The consequences of error can be minor or catastrophic, and through natural selection, creatures with enzymes that do not have the function of such checks have been supplanted by more adaptable ones with various options for matching between amino acid and tRNA. Therefore, in modern cells, synthetase combines with the wrong amino acid on average in one case out of 50 thousand, and with the wrong tRNA only once in 100 thousand attachments.

Some amino acids differ from each other by only a few atoms. If you look at their schemes, it becomes obvious that the likelihood of confusing arginine with alanine is much less than confusing isoleucine for leucine or valine. Therefore, each synthetase that binds to one of the amino acids similar to each other has additional verification mechanisms. Here is an example of such an adaptation in isoleucine-tRNA synthetase:

Each synthase has synthetic center in which an amino acid is attached to a tRNA. The acceptor hairpin of the tRNA captured by the synthetase goes there, as does the amino acid that is ready to react with it. The work of some synthetases ends immediately after the connection of the amino acid and tRNA. But Ile-tRNA synthetase has an increased chance of making mistakes due to the existence of other isoleucine-like amino acids. Therefore, she also has correctional center: from the name it is clear what role it plays in the process of connecting tRNA and amino acids. The figure on the right shows that the position of the end of the tRNA acceptor hairpin in the synthetic center of Ile-tRNA synthetase gives this hairpin an unnatural bend. However, the synthetase holds the tRNA in this position until the amino acid is attached to it. After this connection has occurred, the need to find the acceptor hairpin in the synthetic center is exhausted, and the tRNA straightens out, getting its end with the amino acid attached to it into the correction center.

Of course, the synthetic center also plays a role in screening out amino acids that are not suitable for synthetase. To get into it, the molecule must meet a number of conditions, including having the right size. Despite the fact that leucine and isoleucine contain the same number of atoms, due to differences in the spatial structure, leucine is larger. Therefore, it cannot penetrate into the synthetic center, the size of which corresponds to the more compact isoleucine, and simply bounces off the Ile-tRNA synthetase.
But valine, which is the smallest of these three molecules with a similar atomic structure, easily takes the place of isoleucine in the synthetic center, and synthetase attaches it to tRNA. It is in this case that the correctional center of synthetase comes into play. If the straightening acceptor hairpin is correctly charged and carries isoleucine, then it cannot squeeze inside the correction center: it is simply too small for this molecule. Thus, the straightened tRNA is no longer held by anything, and it is detached from the synthetase. But if valine is attached to tRNA, it slips into the correction center, thereby keeping the tRNA connected to it in the synthetase. Such an excessively long stay of tRNA inside is an error signal for the synthetase, changing its spatial configuration. As a result:
=) valine is detached from tRNA and removed from the synthetase
=) the acceptor hairpin returns to the synthetic site, waiting for attachment to the amino acid
=) synthetase binds to a new amino acid, “charges” tRNA with it and again checks whether isoleucine was used for this.
A similar double recognition mechanism is used by other synthetases.
Physical and chemical properties of DNA
Various factors that break hydrogen bonds (temperature increase above 80 C, changes in pH and ionic strength, the action of urea, etc.) cause DNA denaturation, i.e. change in the spatial arrangement of DNA chains without breaking covalent bonds. The double helix of DNA during denaturation is completely or partially divided into its component chains. DNA denaturation is accompanied by an increase in optical absorption in the UV region of purine and pyrimidine bases. This phenomenon is called hyperchromic effect . Denaturation also reduces the high viscosity inherent in native DNA solutions. When the original double-stranded DNA structure is restored, as a result of renaturation, the absorption at 260 nm by nitrogenous bases decreases due to their "shielding". This phenomenon is called hypochromic effect .
The "unwinding" of each DNA into its component chains is carried out within a certain temperature range. The midpoint of this interval is called the melting point. The melting temperature of DNA depends under standard conditions (a certain pH and ionic strength) on the ratio of nitrogenous bases. G-C pairs containing three hydrogen bonds are stronger, therefore, the higher the content of G-C pairs in DNA, the higher the melting point.
Functions of DNA. In the sequence of nucleotides in DNA molecules, genetic information is encoded. The main functions of DNA are, firstly, to ensure the reproduction of itself in a series of cell generations and generations of organisms, and secondly, to ensure the synthesis of proteins. These functions are due to the fact that DNA molecules serve as a matrix in the first case for replication, i.e. copying information in daughter DNA molecules, in the second - for transcription, i.e. to recode information into the RNA structure.
Rice. 5 Melting curve (DNA denaturation)
Complementary strands of DNA separated during denaturation can, under certain conditions, reconnect into a double helix. This process is called RENATURATION. If denaturation has not occurred completely and at least a few bases have not lost interaction by hydrogen bonds, renaturation proceeds very quickly.
The cytoplasm of cells contains three main functional types of RNA. These are messenger RNAs - mRNAs that act as templates for protein synthesis, ribosomal RNAs - rRNAs that act as structural components of ribosomes, and transfer RNAs - tRNAs involved in the translation (translation) of mRNA information into the amino acid sequence in the protein.
Table 2 shows the differences between DNA and RNA in terms of structure, localization in the cell, and functions.
Table 2 Differences between DNA and RNA
Transfer RNA, tRNA-ribonucleic acid, the function of which is to transport AA to the site of protein synthesis. It has a typical length of 73 to 93 nucleotides and a size of about 5 nm. tRNAs are also directly involved in the growth of the polypeptide chain, joining - being in a complex with an amino acid - to the mRNA codon and providing the conformation of the complex necessary for the formation of a new peptide bond. Each amino acid has its own tRNA. tRNA is a single-stranded RNA, but in its functional form it has a cloverleaf conformation. AA covalently attaches to the 3 "end of the molecule using the enzyme aminoacyl-tRNA synthetase, specific for each type of tRNA. At site C, there is an anticodon corresponding to AA-te. tRNAs are synthesized by ordinary RNA polymerase in the case of prokaryotes and by RNA polymerase III in the case of eukaryotes Transcripts of tRNA genes undergo multistage processing, which leads to the formation of a spatial structure typical of tRNA.
tRNA processing involves 5 key steps:
removal of the 5" leader nucleotide sequence;
removal of the 3'-terminal sequence;
adding a CCA sequence at the 3" end;
excision of introns (in eukaryotes and archaea);
modifications of individual nucleotides.
Transport of tRNA is carried out along a Ran-dependent pathway with the participation of the transport factor exportin t, which recognizes the characteristic secondary and tertiary str-ru of mature tRNA: short double-stranded sections and correctly processed 5 "- and 3" ends. This mechanism ensures that only mature tRNAs are exported from the nucleus.
62. Translation - mRNA codon recognition
Translation is a protein synthesis carried out by ribosomes from amino acids on an mRNA (or and RNA) template. The constituent elements of the translation process: amino acids, tRNA, ribosomes, mRNA, enzymes for aminoacylation of tRNA, protein translation factors (protein factors of initiation, elongation, termination - specific extraribosomal proteins necessary for translation processes), ATP and GTP energy sources, magnesium ions (stabilize ribosome structure). 20 amino acids are involved in protein synthesis. In order for an amino acid to “recognize” its place in the future polypeptide chain, it must bind to a transfer RNA (tRNA) that performs an adapter function. The tRNA that binds to the amino acid then recognizes the corresponding codon on the mRNA. mRNA codon recognition:
The codon-anticodon interaction is based on the principles of complementarity and antiparallelism:
3'----C - G-A*------5' tRNA anticodon
5'-----G-C-Y*------3' mRNA codon
The wobble hypothesis was proposed by F. Crick:
The 3'-base of the mRNA codon has a non-strict pairing with the 5'-base of the tRNA anticodon: for example, Y (mRNA) can interact with A and G (tRNA)
Some tRNAs can pair with more than one codon.
63. Characteristics of the constituent elements of the translation process. Translation (translatio-translation) is the process of protein synthesis from amino acids on the matrix of informational (matrix) RNA (mRNA, mRNA) carried out by the ribosome.
Protein synthesis is the basis of cell life. To carry out this process in the cells of all organisms there are special organelles - ribosomes- ribonucleoprotein complexes built from 2 subunits: large and small. The function of ribosomes is to recognize three-letter (three-nucleotide) codons mRNA, comparing them with the corresponding tRNA anticodons carrying amino acids, and the addition of these amino acids to the growing protein chain. Moving along the mRNA molecule, the ribosome synthesizes a protein in accordance with the information contained in the mRNA molecule.
For recognition of AK-t in the cell, there are special "adapters", transfer RNA molecules(tRNA). These cloverleaf-shaped molecules have a site (anticodon) complementary to an mRNA codon, as well as another site to which the amino acid corresponding to that codon is attached. The attachment of amino acids to tRNA is carried out in an energy-dependent reaction by enzymes aminoacyl-tRNA synthetases, and the resulting molecule is called aminoacyl-tRNA. Thus, the specificity of translation is determined by the interaction between the mRNA codon and the tRNA anticodon, as well as the specificity of aminoacyl-tRNA synthetases that attach amino acids strictly to their corresponding tRNAs (for example, the GGU codon will correspond to a tRNA containing the CCA anticodon, and only AK glycine).
prokaryotic ribosome
5S and 23S rRNA 16S rRNA
34 squirrels 21 squirrels
Prokaryotic ribosomes have a sedimentation constant of 70S, which is why they are called 70S particles. They are built from two different subunits: 30S and 50S subunits. Each subunit is a complex of rRNA and ribosomal proteins.
The 30S particle contains one 16S rRNA molecule and in most cases one protein molecule from more than 20 species (21) . The 50S subunit consists of two rRNA molecules (23S and 5S). It consists of more than 30 different proteins (34), also represented, as a rule, by one copy. Most of the ribosomal proteins perform a structural function.
eukaryotic ribosome
5S; 5,8S and 28S rRNA 18S rRNA
at least 50 proteins at least 33 proteins
The ribosome consists of large and small subunits. The basis of the structure of each subunit is a complexly folded rRNA. Ribosome proteins were attached to the rRNA scaffold.
The sedimentation coefficient of a complete eukaryotic ribosome is about 80 Svedberg units (80S), and the sedimentation coefficient of its subparticles is 40S and 60S.
The smaller 40S subunit consists of one 18S rRNA molecule and 30-40 protein molecules. The large 60S subunit contains three types of rRNA with sedimentation coefficients of 5S, 5.8S, and 28S and 40-50 proteins (for example, rat hepatocyte ribosomes include 49 proteins).
Functional regions of ribosomes
P - peptidyl site for peptidyl tRNA
A - aminoacyl site for aminoacyl tRNA
E - site for the release of tRNA from the ribosome
The ribosome contains 2 functional sites for interaction with tRNA: aminoacyl (acceptor) and peptidyl (donor). Aminoacyl-tRNA enters the acceptor site of the ribosome and interacts to form hydrogen bonds between codon and anticodon triplets. After the formation of hydrogen bonds, the system advances 1 codon and ends up in the donor site. At the same time, a new codon appears in the vacated acceptor site, and the corresponding aminoacyl-t-RNA is attached to it.
Ribosomes: structure, function
Ribosomes are the cytoplasmic centers of protein biosynthesis. They consist of large and small subunits, differing in sedimentation coefficients (sedimentation rate during centrifugation), expressed in units of Svedberg - S.
Ribosomes are present in both eukaryotic and prokaryotic cells, as they perform an important function in protein biosynthesis. Each cell contains tens, hundreds of thousands (up to several million) of these small rounded organelles. It is a rounded ribonucleoprotein particle. Its diameter is 20-30 nm. The ribosome consists of large and small subunits, differing in sedimentation coefficients (sedimentation rate during centrifugation), expressed in Svedberg units - S. These subunits are combined in the presence of a strand of m-RNA (matrix, or informational, RNA). A complex of a group of ribosomes united by a single mRNA molecule like a string of beads is called polysome. These structures are either freely located in the cytoplasm or attached to the membranes of the granular ER (in both cases, protein synthesis actively proceeds on them).
Polysomes of granular ER form proteins that are excreted from the cell and used for the needs of the whole organism (for example, digestive enzymes, proteins of human breast milk). In addition, ribosomes are present on the inner surface of mitochondrial membranes, where they also take an active part in the synthesis of protein molecules.
Transfer RNA (tRNA) plays an important role in the process of using hereditary information by the cell. Delivering the necessary amino acids to the assembly site of peptide chains, tRNA acts as a translational mediator.
tRNA molecules are polynucleotide chains synthesized on specific DNA sequences. They consist of a relatively small number of nucleotides -75-95. As a result of the complementary connection of bases that are located in different parts of the tRNA polynucleotide chain, it acquires a structure resembling a clover leaf in shape (Fig. 3.26).
Rice. 3.26. The structure of a typical tRNA molecule.
It has four main parts that perform different functions. acceptor The "stalk" is formed by two complementary connected terminal parts of the tRNA. It consists of seven base pairs. The 3'-end of this stem is somewhat longer and forms a single-stranded region that ends with a CCA sequence with a free OH group. A transportable amino acid is attached to this end. The remaining three branches are complementary paired nucleotide sequences that end in unpaired sections that form loops. The middle of these branches - anticodon - consists of five pairs of nucleotides and contains an anticodon in the center of its loop. The anticodon is three nucleotides complementary to the mRNA codon, which encodes the amino acid transported by this tRNA to the site of peptide synthesis.
Between the acceptor and anticodon branches are two side branches. In their loops, they contain modified bases - dihydrouridine (D-loop) and the TψC triplet, where \y is pseudouriain (T^C-loop).
Between the aiticodone and T^C branches there is an additional loop, which includes from 3-5 to 13-21 nucleotides.
In general, different types of tRNA are characterized by a certain constancy of the nucleotide sequence, which most often consists of 76 nucleotides. The variation in their number is mainly due to the change in the number of nucleotides in the additional loop. Complementary regions that support the tRNA structure are usually conserved. The primary structure of tRNA, determined by the sequence of nucleotides, forms the secondary structure of tRNA, which has the shape of a clover leaf. In turn, the secondary structure causes a three-dimensional tertiary structure, which is characterized by the formation of two perpendicular double helices (Fig. 3.27). One of them is formed by the acceptor and TψC branches, the other by the anticodon and D branches.
At the end of one of the double helixes is the transported amino acid, at the end of the other is the anticodon. These areas are the most remote from each other. The stability of the tertiary structure of tRNA is maintained due to the appearance of additional hydrogen bonds between the bases of the polynucleotide chain, located in different parts of it, but spatially close in the tertiary structure.
Different types of tRNAs have a similar tertiary structure, although with some variations.
Rice. 3.27. Spatial organization of tRNA:
I - the secondary structure of tRNA in the form of a "clover leaf", determined by its primary structure (the sequence of nucleotides in the chain);
II - two-dimensional projection of the tertiary structure of tRNA;
III - layout of the tRNA molecule in space
APPENDIX (in case someone does not understand this)
Lightning teeth - nucleotides (Adenine-Thymine / Uracil /, Guanine-Cytazine). All lightning is DNA.

To transfer information from DNA, you need to break 2 strands. The bond between A-T and G-C is hydrogen, therefore it is easily broken by the Helicase enzyme:

To prevent knots from forming (As an example, I twisted a towel):



Topoisomerase cuts one strand of DNA at the origin of replication so that the chain does not twist.

When one thread is free, the second can easily rotate around its axis, thereby relieving tension during "unwinding". Nodes do not appear, energy is saved.
Then, an RNA primer is needed to start collecting RNA. A protein that assembles mRNA cannot just assemble the first nucleotide, it needs a piece of RNA to start (it’s written in detail there, I’ll write it out later). This piece is called the RNA primer. And this protein already attaches the first nucleotide to it.


When describing the structure of nucleic acids, different levels of organization of macromolecules are taken into account: primary and secondary structure.
The primary structure of nucleic acids is the nucleotide composition and a certain sequence of nucleotide units in the polymer chain.
Secondary structure of RNA. The ribonucleic acid molecule is built from a single polynucleotide chain.
Secondary structure of RNA
Separate sections of the RNA chain form spiralized loops - "hairpins", due to hydrogen bonds between the complementary nitrogenous bases A-U and G-C. Sections of the RNA chain in such helical structures are antiparallel, but not always completely complementary; they contain unpaired nucleotide residues or even single-stranded loops that do not fit into the double helix. The presence of spiralized regions is characteristic of all types of RNA.
The main role of RNA is direct participation in protein biosynthesis.
Three types of cellular RNA are known, which differ in their location in the cell, composition, size and properties that determine their specific role in the formation of protein macromolecules:
- informational (matrix) RNAs transmit information encoded in DNA about the structure of the protein from the cell nucleus to the ribosomes, where protein synthesis is carried out; the primary structure of all mRNAs, regardless of the uniqueness of their coding sequence, has the same structure of the 5'- and 3'-ends.
So, at the 5'-end there is a modified nucleotide 7-methylguanosine-5'-triphosphate (cap). Several tens of nucleotides separate the cap from the initiation codon, usually the -AUG- triplet. The coding region is followed by one of the termination codons -UGA-, -UUA-, -UAG-. At the 3' end of most mRNAs, there is a nucleotide sequence of 100-200 adenosine monophosphate residues.
- transfer RNAs collect amino acids in the cytoplasm of the cell and transfer them to the ribosome; RNA molecules of this type "learn" from the corresponding sections of the messenger RNA chain which amino acids should participate in protein synthesis.
The spatial structure of any tRNA, regardless of differences in the nucleotide sequence, is described by the universal cloverleaf model. Each tRNA molecule has chain sections that are not involved in the formation of hydrogen bonds between nucleotide residues.
These include, in particular, the site responsible for binding to the amino acid at the 3'-end of the molecule and the anticodon, a specific triplet of nucleotides that interacts complementary with the mRNA codon.
- ribosomal RNA provides protein synthesis of a certain structure, reading information from the information (matrix) RNA. rRNAs form complexes with proteins called ribosomes.
Each ribosome consists of two subunits - small (40S) and large (60S). Ribosome subunits differ not only in the set of rRNAs, but also in the number and structure of proteins.
Publication date: 2015-02-03; Read: 2729 | Page copyright infringement

RNA is a polymer whose monomers are ribonucleotides.
Unlike DNA, RNA is formed not by two, but by one polynucleotide chain (exception - some RNA-containing viruses have double-stranded RNA). RNA nucleotides are capable of forming hydrogen bonds with each other. RNA chains are much shorter than DNA chains.
The RNA monomer - nucleotide (ribonucleotide) - consists of residues of three substances: 1) a nitrogenous base, 2) a five-carbon monosaccharide (pentose) and 3) phosphoric acid. The nitrogenous bases of RNA also belong to the classes of pyrimidines and purines.
Pyrimidine bases of RNA - uracil, cytosine, purine bases - adenine and guanine.
31. Types of RNA and features of its structure
The RNA nucleotide monosaccharide is represented by ribose.
There are three types of RNA: 1) information (matrix) RNA - mRNA (mRNA), 2) transfer RNA - tRNA, 3) ribosomal RNA - rRNA.
All types of RNA are unbranched polynucleotides, have a specific spatial conformation and take part in the processes of protein synthesis.
Information about the structure of all types of RNA is stored in DNA. The process of RNA synthesis on a DNA template is commonly called transcription.

Transfer RNAs usually contain 76 (from 75 to 95) nucleotides; molecular weight - 25,000–30,000.
tRNA accounts for about 10% of the total RNA content in the cell. Functions of tRNA: 1) transport of amino acids to the site of protein synthesis, to ribosomes, 2) translational mediator. About 40 types of tRNA are found in the cell, each of them has a nucleotide sequence characteristic only for it. At the same time, all tRNAs have several intramolecular complementary regions, due to which tRNAs acquire a conformation resembling a clover leaf in shape.
Any tRNA has a loop for contact with the ribosome (1), an anticodon loop (2), a loop for contact with the enzyme (3), an acceptor stem (4), and an anticodon (5). The amino acid is attached to the 3' end of the acceptor stem. Anticodon - three nucleotides that "recognize" an mRNA codon.
It should be emphasized that a particular tRNA can transport a strictly defined amino acid corresponding to its anticodon. The specificity of the connection of amino acids and tRNA is achieved due to the properties of the enzyme aminoacyl-tRNA synthetase.
Ribosomal RNAs contain 3000–5000 nucleotides; molecular weight - 1,000,000–1,500,000.
rRNA accounts for 80–85% of the total RNA content in the cell. In complex with ribosomal proteins, rRNA forms ribosomes - organelles that carry out protein synthesis. In eukaryotic cells, rRNA synthesis occurs in the nucleolus. Functions of rRNA: 1) a necessary structural component of ribosomes and, thus, ensuring the functioning of ribosomes; 2) ensuring the interaction of the ribosome and tRNA; 3) the initial binding of the ribosome and the mRNA initiator codon and the determination of the reading frame, 4) the formation of the active center of the ribosome.
Messenger RNAs are diverse in nucleotide content and molecular weight (from 50,000 to 4,000,000).
The share of mRNA accounts for up to 5% of the total RNA content in the cell. Functions of mRNA: 1) transfer of genetic information from DNA to ribosomes, 2) a matrix for the synthesis of a protein molecule, 3) determination of the amino acid sequence of the primary structure of a protein molecule.
Read also
RNA is a polymer whose monomers are nucleotides.
The three nitrogenous bases are the same as in DNA (adenine, guanine, cytosine); the fourth - uracil - is present in the RNA molecule instead of thymine. RNA nucleotides contain ribose instead of deoxyribose. In the RNA chain...
three main types of RNA: informational(mRNA), or matrix(mRNA), ribosomal(rRNA), and transport(tRNA). They differ in molecular size and function. All types of RNA are synthesized on DNA with the participation of enzymes - RNA polymerases. Messenger RNA makes up 2-3% of all cellular RNA, ribosomal - 80-85, transport - about 15%.
mRNA.
it reads hereditary information from a DNA region and, in the form of a copied sequence of nitrogenous bases, transfers it to ribosomes, where a certain protein is synthesized. Each of the mRNA molecules in the order of nucleotides and in size corresponds to the gene in DNA from which it was transcribed. On average, mRNA contains 1500 nucleotides (75-3000). Each triplet (three nucleotides) on an mRNA is called a codon. It depends on the codon which amino acid will appear in a given place during protein synthesis.
(tRNA) has a relatively low molecular weight of about 24-29 thousand.
D and contains from 75 to 90 nucleotides in the molecule. Up to 10% of all tRNA nucleotides are minor bases, which, apparently, protects it from the action of hydrolytic enzymes. The role of tRNA is that they transfer amino acids to ribosomes and participate in the process of protein synthesis. Each amino acid attaches to a specific tRNA. A number of amino acids have more than one tRNA. To date, more than 60 tRNAs have been discovered that differ in their primary structure (base sequence).
The secondary structure of all tRNAs is presented in the form of a clover leaf with a double-stranded stem and three single-stranded ones). At the end of one of the chains there is an acceptor site - the CCA triplet, to the adenine of which a specific amino acid is attached.
(rRNA). They contain 120-3100 nucleotides. Ribosomal RNA accumulates in the nucleus, in the nucleoli.
Ribosomal proteins are transported to the nucleoli from the cytoplasm, and spontaneous formation of ribosomal subparticles occurs there by combining proteins with the corresponding rRNA. The subparticles of the ribosome are transported together or separately through the pores of the nuclear membrane into the cytoplasm. Ribosomes are organelles 20-30 nm in size.
They are built from two subparticles of different sizes and shapes. At certain stages of protein synthesis in the cell, the ribosomes are divided into subparticles.
Ribosomal RNA serves as a framework for ribosomes and facilitates the initial binding of mRNA to the ribosome during protein biosynthesis.
Question 6 The bonds that form the primary and secondary structures of DNA and RNA. Types of RNA
The genetic code is a way of encoding the amino acid sequence of proteins using a sequence of nucleotides, characteristic of all living organisms.
Properties: 1) genetic code triplet(each amino acid is encoded by three nucleotides); 2) non-overlapping(neighboring triplets do not have common nucleotides); 3) degenerate(with the exception of methionine and tryptophan, all amino acids have more than one codon); 4) universal(mostly the same for all living organisms); 5) in codons for one amino acid, the first two nucleotides are usually the same, and the third varies; 6) has a linear reading order and is characterized by colinearity, t.
e. the coincidence of the order of codons in mRNA with the order of amino acids in the synthesized polypeptide chain.
Publication date: 2014-12-08; Read: 11268 | Page copyright infringement
studopedia.org - Studopedia.Org - 2014-2018. (0.001 s) ...
The cytoplasm of cells contains three main functional types of RNA:
- messenger RNA (mRNA) that act as templates for protein synthesis;
- ribosomal RNA (rRNA) acting as structural components of ribosomes;
- transfer RNAs (tRNAs) involved in the translation (translation) of mRNA information into the amino acid sequence of a protein molecule.
In the nucleus of cells, nuclear RNA is found, constituting from 4 to 10% of the total cellular RNA.
The bulk of nuclear RNA is represented by high-molecular precursors of ribosomal and transfer RNA. Precursors of high molecular weight rRNAs (28 S, 18 S and 5 S RNA) are mainly localized in the nucleolus.
RNA is the main genetic material in some animal and plant viruses (genomic RNA). Most RNA viruses are characterized by reverse transcription of their RNA genome, directed by reverse transcriptase.
All ribonucleic acids are polymers of ribonucleotides connected, as in a DNA molecule, by 3′,5′-phosphorodiester bonds.
Unlike DNA, which has a double-stranded structure, RNA is a single-stranded linear polymeric molecule.
mRNA structure. mRNA is the most heterogeneous class of RNA in terms of size and stability.
tRNA structure.
Transfer RNAs act as mediators (adapters) during mRNA translation. They account for approximately 15% of total cellular RNA. Each of the 20 proteinogenic amino acids has its own tRNA. For some amino acids encoded by two or more codons, there are several tRNAs.
tRNAs are relatively small single-stranded molecules consisting of 70-93 nucleotides. Their molecular weight is (2.4-3.1) .104 kDa.
The secondary structure of tRNA is formed due to the formation of the maximum number of hydrogen bonds between intramolecular complementary pairs of nitrogenous bases.
As a result of the formation of these bonds, the tRNA polynucleotide chain twists with the formation of spiralized branches ending in loops of unpaired nucleotides. The spatial image of the secondary structures of all tRNAs has the shape of a cloverleaf.
Four obligatory branches are distinguished in the "cloverleaf", longer tRNAs, in addition, contain a short fifth (additional) branch.
The adapter function of tRNA is provided by an acceptor branch, to the 3'-end of which an amino acid residue is attached by an ether bond, and an anticodon branch opposite the acceptor branch, at the top of which there is a loop containing an anticodon.
An anticodon is a specific triplet of nucleotides that is complementary in the antiparallel direction to the mRNA codon encoding the corresponding amino acid.
The T-branch carrying the pseudouridine loop (TyC-loop) ensures the interaction of tRNA with ribosomes.
The D-branch, carrying the dehydrouridine loop, ensures the interaction of tRNA with the corresponding aminoacyl-tRNA synthetase.

Secondary structure of tRNA
The functions of the fifth additional branch are still poorly understood; most likely, it equalizes the length of different tRNA molecules.
The tertiary structure of tRNA is very compact and is formed by bringing together individual branches of the clover leaf due to additional hydrogen bonds to form an L-shaped "elbow bend" structure.
Transport RNA, structure and functional mechanism.
In this case, the acceptor arm that binds the amino acid is located at one end of the molecule, and the anti-codon is at the other.

Tertiary structure of tRNA (according to A.S. Spirin)
The structure of rRNA and ribosomes. Ribosomal RNAs form the backbone to which specific proteins bind to form ribosomes. Ribosomes are nucleoprotein organelles that provide protein synthesis from mRNA.
The number of ribosomes in a cell is very large: from 104 in prokaryotes to 106 in eukaryotes. Ribosomes are localized mainly in the cytoplasm, in eukaryotes, in addition, in the nucleolus, in the mitochondrial matrix and in the stroma of chloroplasts. Ribosomes consist of two subparticles: large and small. By size and molecular weight, all the studied ribosomes are divided into 3 groups - 70S ribosomes of prokaryotes (S-sedimentation coefficient), consisting of small 30S and large 50S subparticles; 80S eukaryotic ribosomes, consisting of 40S small and 60S large subunits.
The small subunit of 80S ribosomes is formed by one rRNA molecule (18S) and 33 molecules of various proteins.
The large subunit is formed by three rRNA molecules (5S, 5.8S, and 28S) and approximately 50 proteins.
The secondary structure of rRNA is formed due to short double-stranded sections of the molecule - hairpins (about 2/3 of rRNA), 1/3 - is represented by single-stranded sections rich in purine nucleotides.
Social buttons for Joomla
Proteins form the basis of life. Their functions in the cell are very diverse. However, proteins "can't" reproduce. And all the information about the structure of proteins is contained in genes (DNA).
In higher organisms, proteins are synthesized in the cytoplasm of the cell, and DNA is hidden behind the shell of the nucleus. Therefore, DNA cannot directly serve as a template for protein synthesis. This role is performed by another nucleic acid - RNA.
The RNA molecule is an unbranched polynucleotide with a tertiary structure.
It is formed by one polynucleotide chain, and although the complementary nucleotides included in it are also capable of forming hydrogen bonds between themselves, these bonds occur between the nucleotides of one chain. RNA chains are much shorter than DNA chains. If the content of DNA in a cell is relatively constant, then the content of RNA fluctuates greatly. The greatest amount of RNA in cells is observed during protein synthesis.
RNA plays a major role in the transmission and implementation of hereditary information.
In accordance with the function and structural features, several classes of cellular RNA are distinguished.
There are three main classes of cellular RNA.
- Informational (mRNA), or matrix (mRNA). Its molecules are the most diverse in terms of size, molecular weight (from 0.05x106 to 4x106) and stability.
They make up about 2% of the total amount of RNA in the cell. All mRNAs are carriers of genetic information from the nucleus to the cytoplasm, to the site of protein synthesis. They serve as a matrix (working drawing) for the synthesis of a protein molecule, as they determine the amino acid sequence (primary structure) of a protein molecule.

- Ribosomal RNA (rRNA).
They make up 80–85% of the total RNA content in the cell.
31. The structure of RNA. RNA types, structural features and functions. Secondary structure of tRNA
Ribosomal RNA consists of 3–5 thousand nucleotides. It is synthesized in the nucleoli of the nucleus. In complex with ribosomal proteins, rRNA forms ribosomes - organelles on which protein molecules are assembled. The main significance of rRNA is that it provides the initial binding of mRNA and ribosome and forms the active center of the ribosome, in which peptide bonds are formed between amino acids during the synthesis of the polypeptide chain.
- Transfer RNAs (tRNAs).
tRNA molecules usually contain 75-86 nucleotides. The molecular weight of tRNA molecules is about 25 thousand. tRNA molecules play the role of intermediaries in protein biosynthesis - they deliver amino acids to the site of protein synthesis, that is, to ribosomes. The cell contains more than 30 types of tRNA. Each type of tRNA has its own unique nucleotide sequence.
However, all molecules have several intramolecular complementary regions, due to the presence of which all tRNAs have a tertiary structure resembling a clover leaf in shape.
Secondary structure of RNA- characteristic of tRNA, single-stranded, shaped like a "clover leaf".
Includes:
- relatively short double helixes - stems,
- single-stranded sections - loops.
There are 4 stems (acceptor, anticodon, dihydrouridyl, pseudouridyl) and 3 loops.

"Stem-loop" - an element of the secondary structure of RNA, schematically

"Pseudoknot" - an element of the secondary structure of RNA, schematically

The acceptor stem contains the 3'- and 5'-ends of the polynucleotide chain, the 5'-end ends with a guanylic acid residue, the 3'-end is a CCA triplet and serves to form an ester bond with AA.
The anticodon stem recognizes its codon on mRNA in ribosomes by the principle of complementarity.
The pseudouridyl stem serves to attach to the ribosome.
The dihydrouridyl stem serves to bind to the aminoacyl-tRNA synthetase.
Social buttons for Joomla