The hardness of brass l63 after annealing. Annealing, hardening and heat treatment of brass
Due to the high thermal conductivity, hardenability problems do not arise during hardening heat treatment of copper alloys. With the dimensions of semi-finished products and products used in practice, they are calcined through.
Copper and alloys based on it actively interact with oxygen and water vapor at elevated temperatures, at least more intensively than aluminum and its alloys. In connection with this feature, protective atmospheres are often used in the heat treatment of semi-finished products and products made from copper and its alloys. , while protective atmospheres are rare in aluminum heat treatment technology.
Annealing of copper and its alloys is carried out in order to eliminate those deviations from the equilibrium structure that arose during solidification or as a result of mechanical action or previous heat treatment.
Homogenization annealing consists in heating the ingots to the maximum possible temperature, which does not cause melting of the structural components of the alloys. Segregation phenomena in copper and brass develop insignificantly, and heating of the ingots under hot pressure treatment is sufficient for their homogenization.
The main copper alloys that need homogenization annealing are tin bronzes, since the compositions of the liquid and solid phases in the Cu-Sn system are very different, and therefore intense dendritic segregation develops.
As a result of homogenization annealing, the homogeneity of the structure and chemical composition of the ingots increases. Homogenization annealing is one of the conditions for obtaining a high-quality final product.
Recrystallization annealing is one of the most common technological stages in the production of semi-finished products of copper and alloys based on it.
The onset temperature of copper recrystallization is intensively increased by Zr, Cd, Sn, Sb, Cr, while Ni, Zn, Fe, Co have little effect. The increase in the temperature of the onset of recrystallization with the simultaneous presence of several elements is non-additive, but slightly exceeds the contribution from the most effective impurity. In certain cases, for example, when lead and sulfur are introduced into copper, the total effect is higher than individual effects. Copper deoxidized with phosphorus, in contrast to oxygen-containing copper, is prone to strong grain growth during annealing. The recrystallization threshold in the presence of phosphorus shifts to higher temperatures.
The critical degree of deformation for oxygen-free copper with a grain size of the order of 2*10 in-2 cm after annealing at 800°C for 6 hours is approximately 1%. Impurities, such as iron, increase the critical degree of deformation, which for brass is 5-12% (Fig. 44).
The recrystallization temperature of brasses is also affected by the previous processing, primarily the degree of cold deformation and the grain size formed during this processing. So, for example, the time before the start of recrystallization of brass L95 at temperatures of 440 ° C is 30 minutes at a degree of cold deformation of 30% and 1 minute at a degree of deformation of 80%.
The size of the initial grain affects the crystallization process opposite to the increase in the degree of deformation. For example, in the L95 alloy with an initial grain size of 30 and 15 μm, annealing after 50% deformation at a temperature of 440°C leads to recrystallization after 5 and 1 min, respectively. At the same time, the initial grain size does not affect the recrystallization rate if the annealing temperature exceeds 140°C.
On fig. 45 shows data on the effect of the composition of α-brass on the annealing temperature (degree of deformation 45% annealing time 30 min), which provides a given grain size. Under the same deformation and annealing conditions, with an increase in the zinc content, the grain size decreases, reaches a minimum, and then grows. So, for example, after annealing at 500°C for 30 min, the grain size is: in copper 0.025 mm; in brass with 15% Zn 0.015 mm, and in brass with 35% Zn 0.035 mm. Figure 45 also shows that in α-brass grain begins to grow at relatively low temperatures and grows up to solidus temperatures. In two-phase (α + β)- and special brass, grain growth, as a rule, occurs only at temperatures at which one β-phase. For example, for brass L59, a significant grain increase begins upon annealing above a temperature of 750 ° C.
The annealing temperature of brass is chosen approximately 250–350°C higher than the temperature at which recrystallization begins (Table 16).
During annealing of copper alloys containing 32-39% Zn at temperatures above the α⇔α+β-transition, the β-phase is precipitated, which causes uneven grain growth. It is desirable to anneal such alloys at temperatures not exceeding the α⇔α+β-equilibrium line of the Cu-Zn system. In this regard, brass, which lies in composition near the point of maximum solubility of zinc in copper, should be annealed in furnaces with high temperature control accuracy and high uniformity of its distribution over the volume of copper.
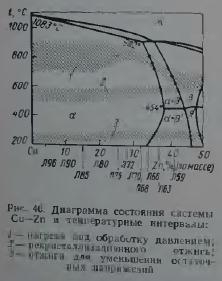
On fig. 46 shows the optimal annealing modes for simple brasses based on the results of summarizing technological recommendations accumulated in domestic and world practice. There is a tendency to increase the temperature of complete annealing of brass with an increase in the content of zinc in them.
When choosing the modes of recrystallization annealing of brasses, it should be taken into account that alloys lying near the α/α+β phase boundary (Fig. 46) can be thermally hardened due to the variable solubility of zinc in copper. Quenching of brasses containing more than 34% Zn makes them prone to aging (Fig. 47), and the ability to harden during aging increases with increasing zinc content up to 42%. This type of thermal hardening of brass has not found practical application. Nevertheless, the cooling rate of L63 type brass after recrystallization annealing affects their mechanical properties. The possibility of decomposition of supersaturated solutions in α-brass containing more than 34% Zn, and in α+β-brass, should also be taken into account when choosing annealing regimes to reduce stresses. Strong cold deformation can accelerate the decomposition of supersaturated α- and β-solutions during annealing.

According to the literature, the temperature of the onset of recrystallization of brass L63 ranges from 250 to 480 ° C. The finest grained structure in the L63 alloy is formed after annealing at temperatures of 300-400 ° C. The higher the degree of previous cold deformation, the smaller the size of the recrystallized grain and the greater the hardness (Fig. 48) under the same annealing conditions.
The quality of the annealed material is determined not only by its mechanical properties, but also by the size of the recrystallized grain. The grain size in a fully recrystallized structure is fairly uniform. Under incorrectly set modes of recrystallization annealing, two groups of grains of different sizes are clearly found in the structure. This so-called double structure is particularly undesirable in deep drawing, bending or polishing and etching operations on the workpiece.
With an increase in grain size to a certain limit, the formability of brass improves, but the surface quality deteriorates. On the surface of the product with a grain size of more than 40 microns, a characteristic roughness "orange peel" is observed.
The stages of the evolution of the deformed structure are significantly extended in time, and therefore it seems possible to obtain a partially or completely recrystallized structure with fine grains by varying the annealing time. Semi-finished products with an incompletely recrystallized structure with a very small grain size are stamped without the formation of an "orange peel".
Incomplete annealing, the duration of which is determined by the degree of preliminary deformation, is carried out in the range of 250-400 ° C. To comply with the exact technological regime, such annealing should be carried out in broaching furnaces, where the operating temperature and holding time (drawing speed) are strictly controlled.
Incomplete annealing is mainly used to reduce residual stresses, which can lead to so-called "seasonal cracking". This type of corrosion, inherent in brass with a content of more than 15% Zn, consists in the gradual development of intergranular cracks under the simultaneous action of stress (residual and applied) and specific chemical reagents (for example, solutions and vapors of ammonia, solutions of mercury salts, wet sulfuric anhydride, various amines etc.). It is believed that the sensitivity of brasses to seasonal cracking is due more to the inhomogeneity of stress than to their absolute value.
The effectiveness of the annealing to reduce the residual stresses is checked by the mercury sample test. The mercury breakdown test method gives a qualitative assessment of the presence of residual stresses. It is based on the different behavior of stressed and unstressed material when exposed to mercury nitrate. During the test, longitudinal and transverse cracks appear on the stressed material, visible to the naked eye. They appear in places of tensile stresses, which can cause the destruction of the product in operation or during storage as a result of corrosion cracking.
Brass annealing modes to reduce residual stresses are given in fig. 46 and in table. 16.
The need for heat treatment.
Heat treatment of steel parts is carried out in cases where it is necessary either to increase the strength, hardness, wear resistance or elasticity of the part or tool, or, conversely, to make the metal softer, easier to machine.
Depending on the heating temperatures and the method of subsequent cooling, the following types of heat treatment are distinguished: hardening, tempering and annealing. In amateur practice, the following table can be used to determine the temperature of a hot part by color.
Heat color: steel |
Heating temperature "C |
Dark brown (visible in the dark) |
530-580 |
brown red |
580-650 |
Dark red |
650-730 |
Dark cherry red |
730-770 |
cherry red |
770-800 |
Light cherry red |
800-830 |
Light red |
830-900 |
Orange |
900-1050 |
Dark yellow |
1050-1150 |
Light yellow |
1150-1250 |
bright white |
1250-1350 |
Hardening gives the steel part greater hardness and wear resistance. To do this, the part is heated to a certain temperature, kept for some time so that the entire volume of the material warms up, and then quickly cooled in oil (structural and tool steels) or water (carbon steels). Typically, parts made of structural steels are heated to 880-900 ° C (hot color light red), from tool steels to 750-760 ° C (dark cherry red color), and from stainless steel to 1050-1100 ° C ( color dark yellow). The parts are heated at first slowly (up to about 500 ° C), and then quickly. This is necessary so that internal stresses do not arise in the part, which can lead to cracks and deformation of the material.
In repair practice, cooling is mainly used in one medium (oil or water), leaving the part in it until it cools completely. However, this method of cooling is unsuitable for parts of complex shape, in which large internal stresses arise during such cooling. Details of a complex shape are first cooled in water to 300-400 ° C, and then quickly transferred to oil, where they are left until completely cooled. The residence time of the part in the water is determined on the basis of: 1 s for every 5-6 mm of the section of the part. In each individual case, this time is selected empirically, depending on the shape and weight of the part.
The quality of hardening depends to a large extent on the amount of coolant. It is important that in the process of cooling the part, the temperature of the coolant remains almost unchanged, and for this its mass must be 30-50 times greater than the mass of the hardened part. In addition, before immersing a hot part, the liquid must be thoroughly mixed in order to even out its temperature throughout the volume.
During the cooling process, a layer of gases forms around the part, which makes it difficult for heat exchange between the part and the coolant. For more intensive cooling, the part must be constantly moved in the liquid in all directions.
Small parts made of low-carbon steels (grades "3O", "35", "40") are slightly warmed up, sprinkled with potassium ferricyanide (yellow blood salt) and again placed in the fire. As soon as the coating is melted, the part is lowered into the cooling medium. Potassium iron-cyanogen melts at a temperature of about 850 ° C, which corresponds to the hardening temperature of these steel grades.
Release of hardened parts.
Tempering hardened parts reduces their brittleness, increases toughness and relieves internal stresses. Depending on the heating temperature, there are low, medium and high tempering.
low vacation used mainly in the processing of measuring and cutting tools. The hardened part is heated to a temperature of 150-250 ° C (tint color is light yellow), kept at this temperature, and then cooled in air. As a result of this treatment, the material, losing brittleness, retains high hardness and, in addition, it significantly reduces the internal stresses that occur during hardening.
Average vacation are used in cases where they want to give parts springy properties and sufficiently high strength with medium hardness. To do this, the part is heated to 300-500 ° C and then slowly cooled.
And finally high vacation subjected to parts in which it is necessary to completely remove all internal stresses. In this case, the heating temperature is even higher -500-600 ° C.
Heat treatment (quenching and tempering) of simple-shaped parts (rollers, axles, chisels, center punches) is often done at one time. The part heated to a high temperature is lowered for some time into the coolant, then removed. The tempering occurs due to the heat stored inside the part.
A small section of the part is quickly cleaned with an abrasive block and the change in tint colors on it is monitored. When a color corresponding to the required tempering temperature appears (220 ° C - light yellow, 240 ° C - dark yellow, 314 ° C - light blue, 330 ° C - gray), the part is again immersed in the liquid, now to full cooling. When tempering small parts (as in quenching), some blank is heated and a tempered part is placed on it. In this case, the tint color is observed on the part itself.
Annealing of steel parts.
To facilitate the mechanical or plastic processing of a steel part, its hardness is reduced by annealing. The so-called full annealing consists in the fact that the part or workpiece is heated to a temperature of 900 ° C, kept at this temperature for some time necessary to heat it throughout the volume, and then slowly (usually together with the furnace) cooled to room temperature.
Internal stresses that have arisen in the part during machining are removed by low-temperature annealing, in which the part is heated to a temperature of 500-600 ° C, and then cooled together with the furnace. To relieve internal stresses and some decrease in the hardness of steel, incomplete annealing is used - heating to 750-760 ° C and subsequent slow (also together with the furnace) cooling.
Annealing is also used in case of unsuccessful hardening or when it is necessary to re-harden the tool for processing another metal (for example, if a copper drill needs to be re-hardened to drill cast iron). During annealing, the part is heated to a temperature slightly below the temperature required for hardening, and then gradually cooled in air. As a result, the hardened part again becomes soft, machinable.
Annealing and hardening of duralumin.
Annealing of duralumin is carried out to reduce its hardness. The part or workpiece is heated to approximately 360 ° C, as in quenching, held for some time, and then cooled in air.
The hardness of annealed duralumin is almost half that of hardened duralumin.
Approximately, the heating temperature of a duralumin part can be determined as follows. At a temperature of 350-360 ° C, a wooden torch, which is carried over the hot surface of the part, is charred and leaves a dark mark. Precisely enough, the temperature of a part can be determined using a small piece of copper foil (the size of a match head), which is placed on its surface. At 400°C, a small greenish flame appears above the foil.
Annealed duralumin has a low hardness, it can be stamped and bent in half without fear of cracking.
hardening. Duralumin can be hardened. During hardening, parts made of this metal are heated to 360-400 ° C, held for some time, then immersed in water at room temperature and left there until completely cooled. Immediately after this, duralumin becomes soft and ductile, easy to bend and forge. He acquires increased hardness after three to four days. Its hardness (and at the same time brittleness) increases so much that it cannot withstand bending through a small angle.
Duralumin acquires its highest strength after aging. Aging at room temperatures is called natural, and at elevated temperatures, artificial. The strength and hardness of freshly hardened duralumin, left at room temperature, increases over time, reaching the highest level after five to seven days. This process is called duralumin aging.
Annealing of honey and brass.Copper annealing. Copper is also subjected to heat treatment. In this case, copper can be made either softer or harder. However, unlike steel, copper is hardened by slow cooling in air, and copper acquires softness by rapid cooling in water. If a copper wire or tube is heated red-hot (600°) over a fire and then quickly immersed in water, the copper will become very soft. After giving the desired shape, the product can again be heated on fire to 400 ° C and allowed to cool in air. The wire or tube will then become solid.
If it is necessary to bend the tube, it is tightly filled with sand to avoid flattening and cracking.
Annealing brass improves its ductility. After annealing, brass becomes soft, easily bent, knocked out and well drawn. For annealing, it is heated to 500°C and allowed to cool in air at room temperature.
Blueing and "blueing" of steel.
Blueing. After burnishing, steel parts become black or dark blue in various shades, they retain a metallic sheen, and a resistant oxide film forms on their surface; protecting parts from corrosion. Before bluing, the product is carefully ground and polished. Its surface is degreased by washing in alkalis, after which the product is heated to 60-70 ° C. Then it is placed in an oven and heated to 320-325 ° C. An even color of the surface of the product is obtained only with its uniform heating. The product treated in this way is quickly wiped with a cloth dipped in hemp oil. After lubrication, the product is again slightly warmed up and wiped dry.
"Blue" steel. Steel parts can be given a beautiful blue color. For this, two solutions are made: 140 g of hyposulfite per 1 liter of water and 35 g of lead acetate (“lead sugar”) also per 1 liter of water. Before use, the solutions are mixed and heated to a boil. Products are pre-cleaned, polished to a shine, after which they are immersed in a boiling liquid and held until the desired color is obtained. Then the part is washed in hot water and dried, after which it is lightly wiped with a cloth moistened with castor or pure machine oil. Parts treated in this way are less susceptible to corrosion.
BRASS
Brasses are the most common copper-based alloys. A summary list of standard brass according to GOST 15527 and their foreign analogues is given in Table. 1.

The state diagram of the alloy of the copper-zinc system is shown in fig. 1

And changes in the temperature of evaporation, melting and casting of copper-zinc alloys depending on the zinc content - in fig. 2.

Change in the modulus of normal elasticity of copper-zinc alloys depending on the zinc content - fig. 3.

The main parameters of the intermetallic phases of the alloys of the system Cu-Zn are given in table. 2.
During the transition from the disordered β-phase to the ordered β '-phase in the specified temperature range, there is a decrease in the coefficient of mutual diffusion and the growth rate of the phase. The activation energy of mutual diffusion in the β'-phase increases, and in the β-phase it decreases with increasing zinc concentration, while itabout 1.5 times more in the β'-phase than in the β-phase. Partial diffusion coefficients of atoms Zn twice as many as Cu atoms in the disordered β-phase and almost coincide with the ordered β'-phase.
Practical applications are simple brass having a phase composition α, α + β, β and β + γ .
The chemical composition of pressure-treated brasses according to domestic ones is given in App. 1.


SIMPLE BRASS
Simple brass, depending on the phase composition, are divided into two types: single-phase α (up to 33% Zn ) and two-phase α + β (over 33% Zn).
In single-phase brasses, in which the zinc content is close to the saturation limit, small amounts of the β-phase are sometimes present as a result of slow diffusion processes. However, inclusions of the /3-phase, observed in very small amounts, do not have a noticeable effect on the properties α -brass. Thus, although these brasses have a two-phase structure, it is advisable to classify them as single-phase brasses in terms of their physical, mechanical and technological properties.
Forming of plain brasses
single phase (A)brass during hot deformation is very sensitive to the content of impurities, especially fusible ( Bi, Pb ). Bismuth in the alloy can segregate along the boundaries, so even a monoatomic layer of it can cause red brittleness in single-phase brasses with a high zinc content. Machinability α - hot brass with increasing zinc content deteriorates. When cold, single-phase brasses work well.
Two-phaseα + β -brasses are processed in a hot state better than single-phase ones due to the presence of high ductility at elevated temperatures β -phases and are less sensitive to impurities. However, they are sensitive to temperature and speed cooling regimes. For this reason, a heterogeneous structure is often observed in hot-pressed semi-finished products. For example, the front end of a bar (strip or pipe) has a predominantly fine needle-like structure and high mechanical properties, while at the rear end of the bar, as a result of chilling, the structure is granular and has reduced mechanical properties.
In a cold state, two-phase brasses are processed worse than single-phase ones. Their plasticity in the cold state depends on the structure. If α -phase is located on the main background of crystals β -phases in the form of thin needles, the workability of two-phase brasses in the cold state improves.
The effect of zinc content in brass on the temperature range of hot working by pressure is shown in fig. 4.

In brass, in the temperature range of 200–600°C, depending on the phase composition and zinc content, a zone of reduced plasticity is observed.
During cold rolling, drawing and deep stamping of brass, regardless of their phase composition, a structure with a grain size of no more than 0.05 mm is preferable.
The total degree of cold deformation of simple brass is determined by a certain limit, above which the ductility drops sharply. This limit of permissible total cold deformation, which decreases with increasing zinc content, is set for each brand of brass.
If we take the highest hot ductility in the homogeneous region β -phase, and at room temperature in the region α -phase beyond 100%, then the machinability of brass by pressure can be quantified ( table. 3).

Such assessments of the machinability of metals and alloys by pressure and other technological characteristics are often used in foreign practice.
Heat Treatment of Plain Brass. The main types of heat treatment of simple brass are recrystallization annealing and stress relief annealing. The process of recrystallization of brasses is determined by the zinc content and phase composition.
Recrystallization start temperature α -brass decreases with increasing zinc content. Recrystallization α -phase in strongly deformed two-phase brass begins at 300°C. Under these conditions, the β-phase remains unchanged and its recrystallization begins at a higher temperature. Therefore, when choosing the annealing temperature to obtain the optimal structure, it is necessary to take into account this feature of two-phase brasses.
The grain sizes of single-phase brasses are determined according to the standards of microstructures (GOST 5362).
During annealing of brass semi-finished products in an air or oxidizing atmosphere, spots are formed on their surface - oxidation products that are difficult to remove during etching. Reducing the oxygen partial pressure (vacuum annealing) prevents staining, but causes a risk of dezincification. Therefore, it is recommended to carry out annealing at a minimum temperature and in a protective atmosphere. Under production conditions, it is most difficult to avoid stains in brasses containing 37-40% zinc.
Machinability of simple brass by cutting. Machinability of brass cutting (turning, milling, planing, grinding) depends on the phase composition of brass. When machining single-phase brass, the chips are long. Two-phase ( A + β ) brass are processed better than single-phase α -brass. With an increase in the content of /3-phase, the chips become more brittle and short. The quantitative assessment of the machinability by cutting simple brass is determined by comparison with brass LS63-3, the machinability of which is taken as 100%. single phase α -brasses are perfectly polished, two-phase ones are somewhat worse. Machinability and polishability of brasses are given in table. 4.

Soldering and welding of simple l brass. Plain brasses are very easily connected with soft solders. Before soldering with soft solder, the surface is cleaned either by grinding or pickling in acid. As a solder, it is preferable to use alloys containing 60% tin. The content of antimony in the solder due to its strong affinity for zinc should be no more than 0.25-0.5%. Soft soldering is preferably performed with chloride fluxes.
single phaseα -brasses are also easily connected by hard soldering, including silver, two-phase A + β - somewhat worse.
Copper-phosphorus solders are self-fluxing, therefore brass soldering with these solders is carried out without fluxes. When soldering with other hard solders, appropriate fluxes must be used.
The lead content in hard solders is limited to 0.5%.
Quantification of the solderability of simple brasses,%: single-phaseα -brass (soft solders) - 100%, single-phaseα -brass (hard solders) - 100%, two-phaseα+ β -brass (soft solders) - 100%, two-phaseα+ β -brass (hard solders) - 75%.
Weldability of simple brasses is somewhat worse than solderability. General quantitative assessment of the weldability of brasses -75% compared to oxygen-free copper taken as 100%. The following types of welding are used to join brass: arc welding with a carbon electrode, arc welding with a consumable electrode, arc welding with a tungsten (non-consumable) electrode in a protective (inert gas) environment, arc welding with a consumable electrode in an inert gas environment, oxy-acetylene, electric contact (spot , roller, butt).
Brass with 20% content Zn poorly amenable to electric contact welding, lighter - brass with 40% Zn . The high zinc content in duplex brass makes arc welding difficult due to zinc evaporation. Therefore, filler materials used in arc welding should contain a relatively small amount of zinc. Brasses containing more than 0.5% Pb are usually difficult to weld. To improve the wettability of the metal during the welding process, preheating to a temperature of 260 ° C is necessary, especially for brass with a high copper content. Carbon electrode welding of brass containing 15-30%, Zn , best done with filler rods (wire) made of Cu alloy + 3% Si . For single-pass welds, copper rods (wire) alloyed with a small amount of tin can be used; for multi-pass welds it is better to use alloy rods Cu + 3% Si.
Brass containing more than 30% Zn , can be welded with a carbon electrode with brass filler rods (wire) Cu + 40% Zn or Cu + 3% Si . To improve the quality of welding, it is necessary to preheat the metal to a temperature of 210°C. As consumable electrodes, wire or rods made of tin-phosphor bronze or aluminum bronze are used.
Arc welding of brass with a tungsten electrode in an inert gas environment is complicated by the release of zinc oxide vapors, which suppress the action of the arc. Therefore, welding should be carried out at high speeds.
Oxy-acetylene welding gives good results. For welding brass with a content of 15-30% Zn it is necessary to use alloy filler rods (wire) Cu + 1.5% Si. Ifoperating conditions of finished products do not cause local corrosion (dezincification), you can use brass with 40% Zn (L60). For welding brass containing more than 30% Zn alloy is used as filler material Cu + 3% Si.
Influence of impurities on the properties of simple brasses. Impurities do not have a significant effect on the mechanical, physical (with the exception of iron, which, at a content of > 3.0%, changes the magnetic properties of brasses) and chemical properties of simple brasses, but significantly affect their technological characteristics. During hot working, single-phase brasses are especially sensitive to fusible impurities.
The quality of products obtained from brass by deep forging depends on the purity of the alloy, therefore, in simple brass intended for deep forging, the content of impurities should be minimal.
Influence of impurities on the quality of semi-finished brass products:
aluminum worsens the quality of the casting, causing foaming in the castings; bismuth causes hot brittleness of brasses, especially single-phase ones; iron hinders the recrystallization process;
siliconimproves soldering and welding processes, increases corrosion resistance; nickel raises the temperature at which recrystallization begins;
leadcauses hot brittleness of brasses, especially single-phase, containing zinc in the range of 30-33%;
antimonynegatively affects the machinability of brass by pressure. Antimony microadditives (<0,1 %) к двухфазным латуням частично локализуют коррозию, связанную с обесцинкованием;
arsenicdegrades the ductility of brasses as a result of the separation of brittle phases at a concentration above its solubility limit: in brasses in the solid state (> 0.1%). Small amounts of arsenic supplements (< 0,04%) предохраняют латуни от коррозионного растрескивания и обесцинкования при контакте с морской водой;
phosphorus refines the structure in the cast state and prevents cracking when heated, accelerates the growth of grains during recrystallization; reduces corrosion associated with dezincification; not recommended as a deoxidizer for copper-zinc alloys;
tinlowers the ductility of brasses and can cause cracking when heated if the iron content is > 0.05%.
Brass Modification carried out by introducing into the melt:
additives of elements that form refractory compounds, which, if structurally consistent, will serve as centers of crystallization;
surface-active metals, which, concentrating on the faces of nascent crystals, slow down their growth.
As modifiers in brass, elements such as iron, nickel, manganese, tin, yttrium, calcium, boron, and mischmetal are used.
Corrosion properties of brasses. Brass has satisfactory resistance to industrial, marine and rural atmospheres. They fade in the air. Corrosive effect on brass containing >15% zinc, exert carbon dioxide and halogens.
Brass containing <15% Zn , in their corrosion resistance are close to industrial purity copper.
Under the influence of oxidizing acids, brass corrodes intensively. The limiting concentration of nitric acid, at which no noticeable corrosion is observed, is 0.1% (by weight). Sulfuric acid acts less aggressively on brass, however, in the presence of oxidizing salts K 2 SG 2 ABOUT 7 And Fe 2 (S0 4) 3the corrosion rate increases by 200-250 times. Of the non-oxidizing acids, hydrochloric acid has the strongest corrosive effect.
Corrosion resistance of brasses in relation to most acids that do not have an oxidizing ability is satisfactory. Brass is also resistant to dilute hot and cold alkaline solutions (with the exception of ammonia solutions) and cold concentrated neutral salt solutions. Brass is inert to river and salt water. Upon contact with river water containing a small amount of sulfuric acid, and in sea water, simple brasses noticeably corrode. The corrosion rate depends on the temperature, concentration, degree of contamination and the speed of the flow around the metal surface. In relation to the soil, brass has good corrosion resistance, and is neutral to food products. The corrosion rate of brass in soil ranges from 0.0005 mm/year (in loamy soil with pH 5.7) to 0.075 mm/year (in ash soil with pH 7,6).
Dry gases - fluorine, bromine, chlorine, hydrogen chloride, hydrogen fluoride, carbon dioxide, oxides of carbon and nitrogen at a temperature of 20 ° C and below have practically no effect on brass, however, in the presence of moisture, the effect of halogens on brass increases sharply; sulfur dioxide causes corrosion of brass at its concentration in the air - 1% and air humidity > 70%; hydrogen sulfide significantly affects brass under all conditions, however, brass containing Zn > 30% more resistant than brass with low zinc content.
Fluorinated organic compounds, such as freon, have practically no effect on brass.
In wet saturated steam at high speeds (about 1000 m 3 / c ) pitting corrosion is observed; therefore, brass is not used for superheated steam.
Corrosion resistance of brasses in various environments is given in table. 5.

In mine waters, especially in the presence of Fe 2 (SO 4 ) 3 brass corrodes heavily. The fluoride salts present in the water have a weak effect on brass, the chloride salts have a stronger effect, and the iodine salts have a very strong effect.
Brass, in addition to general corrosion, is also subject to special types of corrosion: zinc coating and "seasonal" cracking.
Dezincification is a special form of corrosion in which a solid solution of zinc in copper dissolves and copper is electrochemically deposited at cathode sites. Zinc corrosion products can be removed or retained in the form of an oxide film. The solution in which brass is subjected to dezincification usually contains more zinc than copper.
As a result of dezincification, brass becomes porous, reddish spots appear on the surface, and mechanical properties deteriorate. Dezincification is observed when brass comes into contact with electrically conductive media (acidic and alkaline solutions) and manifests itself in two forms: continuous and local. The dezincification process intensifies with an increase in the zinc content, as well as with an increase in temperature and aeration. Single phase brass containing >15% Zn , undergo dezincification in acidic solutions (nitrates, sulfates, chlorides, ammonium salts and cyanides). In two-phase brass, the dezincification process is markedly enhanced and can occur even in aqueous media. The most vulnerable isβ-phase.
Small additions of arsenic, phosphorus and antimony partially localize corrosion associated with dezincification. Arsenic and antimony protect against dezincification mainlyα -phase.
"Seasonal" or intergranular cracking is observed in brass as a result of exposure to corrosive agents in the presence of tensile stresses. Corrosive agents include: vapors or solutions of ammonia, condensates with sulfurous gases, wet sulfuric anhydride, solutions of mercury salts, various amines, components of pickling solutions, wet carbon dioxide. If the atmosphere contains traces of ammonia, wet carbon dioxide, sulfur dioxide, and other corrosive agents, then "seasonal" cracking occurs with temperature fluctuations, as a result of which condensation of corrosive agents occurs on the surface of the parts.
Brass containing up to 7% zinc is not very sensitive to "seasonal" cracking. In brass containing 10 to 20% zinc, intergranular cracking is not observed if the internal tensile stresses do not exceed 60 MPa. Zn , undergo stress corrosion cracking only in the cold-deformed state in an aqueous solution of ammonia. The most prone to stress corrosion cracking are single-phase brasses with a zinc concentration close to the saturation limit, and two-phase brasses. They are resistant to "seasonal" cracking only in the presence of tensile stresses.< 10 МПа.
The susceptibility to corrosion cracking of copper-zinc alloys in ammonia vapor is shown in fig. 5.

To prevent corrosion cracking of brass, it is necessary to apply low-temperature annealing and protect them from oxidation during storage. To relieve internal stresses, pre-recrystallization annealing is performed.
To protect brass from oxidation, it is recommended to passivate them in the following media: a slightly acidic aqueous solution containing about 6% chromic anhydride and 0.2% sulfuric acid; aqueous solution containing 5 % chromic and 2% chrome alum.
Brass is also protected with corrosion inhibitors such as benzotriazole or toluenetriazole. Benzotriazole forms a film on the surface (< 5 нм), которая предохраняет латуни от коррозии в водных средах, различных атмосферах и других агентах. Коррозионные ингибиторы могут быть введены в состав лаков и защитной оберточной бумаги.
In the case of electrochemical corrosion, brass, in contact with various metals and alloys, manifests itself in two ways: in some cases, the anode, in others, the cathode ( tab. 6 ).

When brass comes in contact with silver, nickel, cupronickel, copper, aluminum bronze, tin and lead, electrochemical corrosion does not occur.
Brass oxidizes when heated. The rate of oxidation of brass increases exponentially with increasing temperature, doubling approximately every 360K. At temperatures above 770K, evaporation of zinc is most intense if its concentration in the alloys exceeds 20 %.
The change in some of the physical and mechanical properties of brass depending on the zinc content is shown in fig. 6-9.




Typical physical, mechanical and technological properties of brass are given in P ril. 2, 3, 4.



Special pressure treated brass
Special or multi-component brasses are copper-zinc alloys of complex compositions in which the main alloying elements are aluminum, iron, manganese, nickel, manganese, nickel, silicon, tin and lead. These elements, as a rule, are introduced into brass in such quantities that they are completely dissolved inα andβ phases. In addition to these elements, small additions of arsenic, antimony and other elements are introduced into brass.
The influence of alloying elements is manifested in two ways: phase properties change (Aand/3) and their relative amounts, i.e. boundary of phase transformations.
To determine the boundaries of phase transformations in the system or the "apparent" ("fictitious") copper content when adding an alloying element, an empirical equation is used:
A ’ = A *100/(100+ X *(K e-1)),
Where A'- apparent (fictitious) content of copper, % (by weight); A -actual copper content, % (by weight); X- the content of the third component, % (by weight); Ke- Guinier coefficient characterizing the influence of the alloying element on the phase composition (at K e> 1, the number ofβ'-phase).
Meaning Kefor various elements: for Ni K uh from -1.2 to -1.4, for co K e=-1, for Mn K e=0.5, for Fe K e=0.9, for Pb K e=1, for Sn K e=2, for Al K e=6, for Si K e from 10 to 12.
Lead brass
Lead brass - copper-zinc alloys alloyed with lead. System State Diagram Cu-Zn-Pb featured on rice. 10.

The solubility of lead in alloys in the solid state is negligible. In two-phase copper-zinc alloys (containing Zn 40%) lead solubility at 750°С inβ -phase a little more than 0.2%; Lead is practically insoluble at room temperature. In two-phase brass (in equilibrium), lead is located insideα Andβ -phases and partially at the boundaries of these phases. Lead, when released along the boundaries of phases or grains, significantly worsens the deformability of brass in the hot state.
Lead in alloys A + β performs a dual role: on the one hand, it is used as a phase that promotes chip breaking, on the other - as a lubricant that reduces the coefficient of friction during cutting. The effectiveness of lead additives is determined by its amount and the structure of the alloy, the size and nature of the distribution of lead particles, grain size a -phase, quantity and distributionβ-phases.
By improving machinability, lead significantly reduces the impact strength of brasses, impairs machinability by pressure, soldering and welding, polishability, and complicates the galvanic surface treatment of products.
The strength characteristics of lead brass decrease more intensively with increasing temperature compared to simple brass. The tensile strength of brasses containing about 2% lead at a temperature of 600°C is 10 MPa, at a temperature of 800°C - practically equal to zero.
Depending on the processing of finished deformed semi-finished products, lead brass is classified into three main types: for cold working by pressure, for hot stamping, for processing on automatic lathes.
Structure lead visty la tuney. processed by cold pressure state, consists ofα -phase and lead, the content of which should be within such limits as to ensure high machinability. These alloys include brass grades LS74-3, LS64-2, JIC 63-3 and LS63-2.
Lead e lat un and hot-pressurized condition and intended for hot forging and stamping - two-phase (α +β). The zinc content in brass should be such that the transformation α + β cleanβ -phase occurred completely and at a relatively low temperature.
Estimated content β -phase is about 20%. The lead content is from 1 to 3%. These brasses include lead brass grades LS60-1, LS59-1 and LS59-3. Lead e no lat. used for machining on automatic lathes and in microtechnology (i.e. for the manufacture of parts that are very small in size, on the order of 1 mm) - two-phase, with a high lead content; LS63-3 (low/3-phase) and LS58-3 (high β -phases).
Brass used in microtechnology is subject to special requirements for the uniformity of the chemical composition, tolerances for the main components and microstructure (size and distribution of lead particles, quantity and distribution β -phases, grain size α -phases). The homogeneity of the chemical composition (homogeneity of the alloy) must be ensured in small areas.
The boundaries of the optimization of the microstructure of lead brass for "micro-details" are determined by the content β -phases from 10 to 30%, grain size α -phase - from 10 to 50 microns with an average lead particle diameter of 1-5 microns.
Processing of lead brass. Oxides of various elements impair the machinability of lead brass, therefore, when they are melted and cast, careful control over their content is necessary. Of the impurity elements, iron has the most negative effect on machinability, so its content is subject to special restrictions. Casting is carried out in two ways: in molds and semi-continuous (continuous) method. To achieve stability of the chemical composition, it is preferable to cast lead brass in a continuous (semi-continuous) way.
Lead does not affect the temperature and the crystallization process of copper-zinc alloys, it solidifies at 326°C and, in the case of precipitation along grain (phase) boundaries, worsens the hot deformability of two-phase alloys.
The composition areas of standard hot and cold processed lead brass are shown in fig. eleven.

When hot stamping lead brass containing 56-60% Cu (LS59-1), the tendency to cracking is determined mainly by the deformation temperature. The optimal temperature range at which cracks do not form is quite narrow and is in the temperature range that makes up the lines on the state diagram Cu-Zn , delimiting the two-phase α + β Andsingle-phaseβ - areas.
The content of lead, as well as low-melting impurities (bismuth, antimony, and others) does not affect the tendency to cracking during hot stamping of two-phase lead brass (α + β ).
The influence of the chemical composition on the machinability and pressure of lead brass is shown in table. 7.

Leadα -brasses are processed in a cold state, however, under certain conditions, hot pressing is also possible.
The main types of heat treatment of lead brass are full recrystallization annealing and low-temperature annealing to relieve internal stresses.
Leaded brasses are worse than simple brasses, soldered, welded and polished. For joining lead brass, it is not recommended to use oxy-acetylene welding, arc welding in a shielding gas environment and arc welding with a consumable electrode.
Co. corrosion resistance of lead brass . Lead brass has: excellent resistance to pure hydrocarbons, freon, fluorinated hydrocarbon coolants and varnishes; good resistance to industrial, marine, rural atmospheres, alcohols, diesel fuel and dry carbon dioxide; moderate resistance to crude oil and aqueous carbon dioxide; poor resistance to ammonium hydroxide, hydrochloric and sulfuric acids.
Tin yanny la t uni
Tin slightly affects the change in the boundaries of phase transformations, however, it significantly changes the nature β -phases. System State Diagram Cu-Zn-Sn shown on rice. 12.

Duplex tin brasses have high corrosion resistance in many environments. With an increased content of tin in brass, a new phase γ appears. The γ phase is a brittle component that significantly impairs the cold workability of brass. Appearance γ -phases in two-phase brass (a +/3) is observed when the content of tin is over 0,5% (if the tin content exceeds this limit, then during the transformation β a δ-phase is released, enveloping α -phase. The appearance of brittle phases limits the alloying of brass with tin. Tin content over 2% in brass impairs their hot workability. Standard tin brass can be divided into two types: single-phase (α - solid solution) and three-phase ( α + β + γ ).
Aluminum brass
Aluminum brass - copper-zinc alloys, in which the main alloying additive is aluminum.
Aluminum, due to its high Guinier coefficient (Ke = 6) and significant solubility in the solid state, compared with other elements (except silicon), even in small quantities, has a noticeable effect on the properties of brass. Aluminum additives increase the mechanical properties and corrosion resistance of brasses, but somewhat worsen their ductility. The amount of introduced aluminum is limited to the limits above which brittle γ -phase ( rice. 13).

With copper content, % (by weight): 70; > / J 65; 60 limiting aluminum content, % (by weight): 6; 5 and 3, respectively. In pressure-treated brasses, the aluminum content does not exceed 4%, in foundry high-strength brasses 7%.
Alloying of brasses is carried out with aluminum alone or in certain proportions with other elements (iron, nickel, manganese and etc.).
One aluminum, as a rule, is alloyed with single-phase brasses (LA85-0.5, LA77-2). For containment of dezincification and prevention of stress corrosion cracking in contact with sea water in single-phase aluminum brasses containing more than 15% Zn, 0.02-0.04 As (LAMsh77-2-0.05) is introduced.
An excess of arsenic (> 0.062%) impairs the ductility of brasses. Aluminum together with iron (LAZH60-1-1) and nickel (LAN59-3-2) is introduced mainly into two-phase brass.
Iron improves the ductility of lead-containing brasses; when hot, it refines the structure and improves their mechanical properties; Nickel improves corrosion resistance. Iron and nickel somewhat reduce the cold ductility of brass.
Alloying brasses with aluminum, nickel and small additions of manganese and silicon (LANKMts75-2-2.5-0.5-0.5) makes them precipitation hardening and significantly improves mechanical properties, especially elastic characteristics.
Single-phase aluminum brasses are satisfactorily processed by pressure in the hot state and well - in the cold; two-phase - good in a hot state and satisfactorily in a cold one. Machinability varies from 30 to 50% (compared to LS63-3 brass).
Aluminum brass, compared to lead, is worse connected with solders, but welds somewhat better; in terms of polishability, they are close to two-phase simple brasses ( tab l. 8).

Ferrous brasses
Iron additives significantly refine the structure of brass, thereby improving mechanical properties and technological characteristics. However, the "alloys of the system Cu-Zn-Fe rarely applied. Distribution received multicomponent brass.
manganese brass
Alloying brass with manganese significantly increases their corrosion resistance in contact with sea water, chlorides and superheated steam.
System Alloy State Diagram Cu-Zn-Mn shown in fig. 14.

Manganese additives have little effect on the structure of brasses. However, manganese reduces the stability of the ordered phase lattice β . When the content of Mn > 4.7% (at.) in the alloy, a partially disordered state is observed at a quenching temperature of 520°C.
Manganese has the most favorable effect on the properties and technological characteristics of brass in combination with other alloying elements (aluminum, iron, tin, nickel).
Silicon brasses
Silicon in the solid state is soluble in brass in significant quantities, but its solubility decreases with increasing zinc content. Solid solution region Aunder the influence of silicon and zinc, it shifts sharply towards the copper corner (Fig. 15 ) .

With an increase in the silicon content in the alloy structure Cu-Zn-Si a new phase appears Tohexagonal syngynia, which is plastic at elevated temperatures and, unlike β -phase is polarized. As the temperature decreases (below 545°С), the eutectoid decomposition of the c-phase intoα + γ ".
Silicon brass containing 20% Zn and 4% Si not suitable for pressure treatment due to low plasticity. To obtain deformed semi-finished products, silicon brass containing<4% Si.
Small additives of silicon improve the technological characteristics of brass during casting and hot forming, increase mechanical properties and anti-friction properties.
Nickelbrass
Alloying brasses with nickel increases their mechanical properties and corrosion resistance. Nickel brass is more resistant to dezincification and stress corrosion cracking than other brasses.
As can be seen from the state diagram of the alloy system Cu-Zn-Ni (rice. 16), nickel has a noticeable effect on the structure of brass, expanding the area of solid solution α

When alloying with nickel, some two-phase brasses can be converted into single-phase ones.
Alloying of brass L62 with nickel in an amount of 2-3% (by weight) makes it possible to obtain a single-phase alloy with fine grains, high and uniform mechanical properties and increased corrosion resistance. Due to the addition of nickel in the production of deformed semi-finished products, the occurrence of such a negative phenomenon as a line structure is excluded.
Recommendations for improving the properties of copper-zinc alloys based on foreign experience. The properties of brass, along with the purity of the initial alloy components, the methods and modes of melting and casting, are greatly influenced by the modes of their processing and preparation of the charge.
To reduce the formation of porosity and bubbles in sheets (strips) and strips made of brass grades L70, L68, L63 and L60: avoid contamination of the charge with phosphorus; waste in the form of shavings containing oil, emulsion, etc., be subjected to oxidative roasting before melting; add copper oxide to the melt in the amount of 0.1-1.0 kg per 100 kg of charge; pay special attention to the optimal modes of casting and hot rolling; anneal hot-rolled strips before cold rolling.
To increase the resistance of brasses L68 and L70 to corrosion cracking, it is necessary to pay great attention to the selection of the cold rolling and annealing regime. The total reduction during the last cold rolling should be more than 50%, the optimum annealing temperature is 260-280°C.
To increase the resistance of two-phase brass to dezincification (and this is possible if the proportion β -phase in the structure of the alloy is about 30%), it is necessary to carry out heat treatment in the temperature range of 400-700°C (depending on the composition of the alloy).
To prevent dezincification of L63 brass and obtain a high-quality surface during light annealing (in bell and shaft furnaces), the recrystallization annealing temperature is maintained within 450-470°C. At this temperature, within 1-4 hours, a strip (tape) is obtained with a grain size of 0.035-0.045 mm, a tensile strength of 33-35 kgf/mm 2 and a relative elongation of 50%.
Tempering the metal allows you to make some changes in its structure, making it softer or vice versa hard. When hardening, a lot depends not only on the heating itself, but also on the process and time of cooling. Basically, manufacturers harden steel, making the product more durable, however, copper can also be hardened if the need arises.
Copper tempering - manufacturing process
Copper is hardened using the annealing method. During heat treatment, copper can be made softer or harder, depending on what it will be used for. However, it is important to remember that the way copper is quenched is significantly different from the way steel is quenched.
Hardening of copper occurs during slow cooling in air. If it is necessary to obtain a softer structure, then hardening is carried out by rapidly cooling the metal in water immediately after heating. If you want to get a very soft metal, then you should heat the copper to red (this is about 600 °), and then lower it into water. After the product has gone through the deformation process and acquired the desired shape, it can be heated again to 400 °, and then allowed to cool in the air.
Copper Hardening Plant
Hardening of copper is carried out in special equipment designed for this. There are several types of hardening machines, but induction equipment has become the most popular today. The induction machine is excellent for hardening copper, allowing you to get a high quality product. Thanks to the automated software of the HDTV equipment, it is configured with high accuracy, which indicates the heating time, temperature, as well as the method of cooling the metal.
If an enterprise constantly hardens metal products, then it would be best to pay attention to a special set of equipment designed for comfortable fast hardening. The hardening complex ELSIT has all the necessary equipment for high frequency hardening. The set of the hardening complex includes: an induction unit, a hardening machine, a manipulator and a cooling module. If the customer needs to harden products with different shapes, then a set of inductors of various sizes can be included in the package of the hardening complex.
Graaver 04-03-2010 20:17
I'll start from afar.
I have been manufacturing sports medals for more than ten years, but there are questions that I constantly encounter, but I have not found out the final answers to them .. can anyone help? here is one of them..
To increase plasticity, when pressing, the brass billet must be annealed .. and here the fun begins ..
At the moment I use the following recipe for annealing brass L63 (experimentally derived):
Heating in the oven to t=560 C, holding for 1.5-2 hours, cooling in air..
With the same parameters (brand of brass, maintenance mode), the output is a completely different result.
In one case, all "chiki-bunches" .. brass becomes "soft", easily deformed and has an even, mirror-smooth surface (corresponding to the "mirror" of the stamp).
In another version, everything seems to be the same .. "soft" (plastic), only where there should be a "mirror", a light, barely noticeable "cellulite-orange peel" appears .. it seems like a trifle, but horror is not pleasant
The question is..
Maybe someone faced a similar problem, how is it solved?
Interested in - temperature, exposure time during heating and time (method) of cooling ..
Also, is it possible to "cure" brass billets "infected with cellulite" (not the correct MOT)?
With all respect, Andrew.
Ress75 04-03-2010 20:47
In jewelry techniques, there is such a technique: it is called r .. (I don’t remember longer). The meaning is repeated annealing (6 times) of silver, etc. The metal begins to shove from the inside of the product and with each cycle the surface of the product swells locally - such a desert relief comes out with orange peel. In general, it’s beautiful. Further, it naturally faded, etc. Maybe something similar comes out here?
Yuzon 04-03-2010 21:45
Precisely the whole L 63? or maybe LS
Graaver 04-03-2010 22:08
quote: And brass from one party, or different deliveries?
Precisely the whole L 63? or maybe LS
Party one..
Sometimes they cut three sheets (even if we assume that the sheets are different, all the blanks are brought in one bag, this is about 900 pieces, 300 pieces / sheet.), I anneal .. part is normal, part is "cellulite" (i.e. one batch after MOT is all normal, another problem)..
True, I admit that the exposure time in the oven is different ..
Problems with the temperature difference are excluded .. the oven allows you to keep the temperature "+" _ "-" 1gr.
There is no "cellulite" without annealing, but oh, how hard it is to push through such a workpiece ..
If someone has experienced this, .. can there be a guaranteed recipe?
So that both "soft" and without "cellulite" ..?
Graaver 04-03-2010 22:19
Maybe someone knows under what conditions (exceeding what parameters) this muck occurs?
sm special 04-03-2010 23:35
Perhaps "google" on a request for brass annealing defects can clarify something ...
Yuzon 05-03-2010 11:53
You can also try:
You don’t need to do a long exposure, according to the process: at t = 600 C, loading, warming up about 1 mm / min. as the temperature leveled off, so cooling in air or through water.
IMHO: With a long exposure in an oxidizing atmosphere, zinc begins to oxidize and "rushes" the surface.
And sometimes sheet distributors are to blame (they can’t stand their own process)
Graaver 05-03-2010 14:41
When experimenting with t = 600 C, I was guaranteed to get "cellulite", although the exposure time was long ..
In the near future there will be an opportunity to experiment again ..
I'll try to reduce the time the blanks are in the oven ..
Nestor74 05-03-2010 16:39
2Graaver
after the holidays I’ll check with my people (the guys work a lot with brass - souvenirs, premium paraphernalia), maybe they’ll tell me something, I’ll unsubscribe if by that time this issue is still relevant.
Yuzon 05-03-2010 16:50
quote: I'll try to reduce the time the blanks are in the oven ..
On time: the less the better. just to get the oven up and running.
Do not ship in a tight pack.
Boole 05-03-2010 17:28
you can, your 5 kopecks: immediately into the water, without exposure to air
Boole 05-03-2010 17:29
simple rolling of copper alloys is directly opposite to the TO of steels - ductility rises
Graaver 05-03-2010 20:12
quote: after the holidays I’ll check with my people (the guys work a lot with brass - souvenirs, award paraphernalia), maybe they’ll tell me something, I’ll unsubscribe if by that time this issue is still relevant.
Any advice is appreciated!
And practical experience is especially important!
quote: load at 600 and transfer the oven to t=560.
Do not ship in a tight pack.
I tried cooling in water .. but again, the exposure of the blanks in the furnace was significant, and in the batch everything was as “tight” as possible ..
This must have been the reason for the failure.
Graaver 12-03-2010 19:52
What happened was the least expected.
The story in a nutshell is...
I ordered two sheets of brass, without checking I gave it to production ..
It turned out that one sheet, as ordered, was brass (L63), and the second was bronze (the brand is unknown, it has a pleasant pink tint) ..
Bronze does not suit me for those. characteristics.
Therefore, the whole party, in order not to disappear idle, moves to a flea market.
Who might need it?
Here is a photo of blanks and a "trial" medal from this material.
Graaver 13-03-2010 09:27
I conducted an experiment with a new batch .. the "minimum required" holding time in the oven + "loose" load + cooling in water ..
The experiment was a success.. "cellulite" is missing!
Many thanks to the one-palatniks "Bul" and "UZON" for good advice !!!
I apologize for being annoying..
Is it possible to "restore" brass after a wrong MOT?
With all respect, Andrew.





